Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: The monoclonal antibody bimekizumab potently and selectively neutralizes both IL-17A and IL-17F. We report the 48-week efficacy and safety of bimekizumab in patients (pts) with active ankylosing spondylitis (AS; NCT02963506). The positive primary endpoint (Assessment in SpondyloArthritis International Society [ASAS] 40 response rate at Week 12) was previously reported.1
Methods: Eligible pts with active AS (Bath AS Disease Activity Index [BASDAI] ≥4; spinal pain ≥4 [0–10]) who fulfilled the modified New York criteria (central reading) with inadequate response/intolerance to NSAIDs were randomized 1:1:1:1:1 to subcutaneous (sc) bimekizumab 16 mg, 64 mg, 160 mg, 320 mg, or placebo every 4 weeks, for 12 weeks (double-blind period). Subsequently, pts in the 16 mg, 64 mg, and placebo groups were re-randomized 1:1 to sc bimekizumab 160 mg or 320 mg every 4 weeks through Week 48; dosing in the original 160 mg and 320 mg groups was unchanged through Week 48 (dose-blind period). Efficacy endpoints included ASAS20, ASAS40, ASAS5/6, ASAS partial remission, and AS Disease Activity Score with C-reactive Protein (ASDAS-CRP) to Week 48; data presented for all pts who began the dose-blind period and received ≥1 dose of study drug. Non-responder imputation accounted for missing binary scores; multiple imputation accounted for missing continuous values.
Results: Of 303 randomized pts, 265 (87.5%) completed the 48-week treatment period. Pts (mean [SD] age 42.2 [11.8] years; males 84.5%; median [range] time from first symptoms 12.3 [0.2−47.2] years; mean [SD] BASDAI 6.5 [1.4]; mean [SD] total spinal pain 7.1 [1.7]; HLA-B27 positive 89.1%; prior anti-TNF therapy 11.2%) had similar baseline characteristics across treatment groups.
At Week 12, significantly more bimekizumab-treated pts vs placebo achieved ASAS40 (16 mg 29.5%; 64 mg 42.6%; 160 mg 46.7%; 320 mg 45.9%; placebo 13.3%; p< 0.05, all doses). ASAS40 response rates increased to Week 24 and were maintained to Week 48 (35.5–64.0%; Table 1). Improvements observed at Week 12 in other efficacy endpoints were sustained to Week 48: ASAS20 51.9–80.0%, ASAS5/6 41.9–80.0%, ASAS partial remission 20.6–34.4% (Table 1). At Week 12, mean improvement from baseline in ASDAS-CRP scores were from –0.3 to –1.7; scores improved further from baseline and were maintained to Week 48: from –1.6 to –2.0 (Table 1).
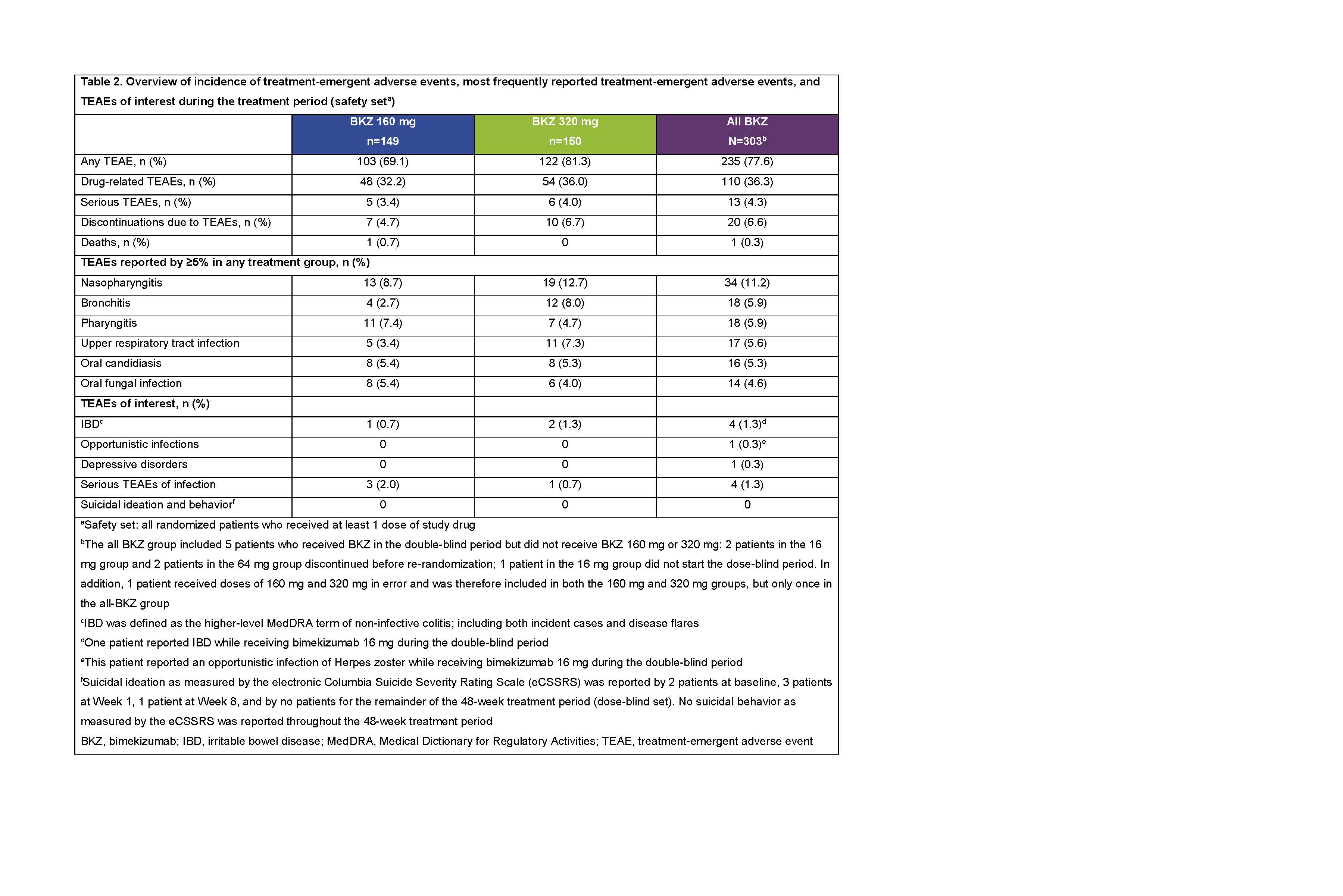
During the 48-week treatment period, treatment-emergent adverse events (TEAEs) were reported by 235/303 (77.6%) pts; 20/303 (6.6%) discontinued due to TEAEs (safety set; Table 2). One death (cardiac arrest, 160 mg group, double-blind period) was judged unrelated to study drug by the investigator. Most commonly reported TEAEs were nasopharyngitis, bronchitis, and pharyngitis. Irritable bowel disease was reported by 4/303 (1.3%) pts; no TEAEs of suicidal ideation were reported. Serious infections were reported by 4/303 (1.3%) pts (Table 2). p< 0.05
Conclusion: Following positive results at Week 12,1 this Phase 2b study demonstrated the sustained efficacy of bimekizumab in pts with active AS to Week 48. Bimekizumab was generally well tolerated and no new or unexpected safety findings were identified.
References: 1van der Heijde ARD 2018;77(Suppl.2):A70

AS0008 Wk48_van der Heijde_ACR19 abstract_Table 1
AS0008 Wk48_van der Heijde_ACR19 abstract_Table 2
BKZ 160 mg
To cite this abstract in AMA style:
van der Heijde D, Gensler L, Deodhar A, Baraliakos X, Poddubnyy D, Kivitz A, Oortgiesen M, Baeten D, Goldammer N, Coarse J, Farmer M, Dougados M. Dual Neutralization of IL-17A and IL-17F with Bimekizumab in Patients with Active Ankylosing Spondylitis: 48-Week Efficacy and Safety Results from a Phase 2b, Randomized, Blinded, Placebo-Controlled, Dose-Ranging Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/dual-neutralization-of-il-17a-and-il-17f-with-bimekizumab-in-patients-with-active-ankylosing-spondylitis-48-week-efficacy-and-safety-results-from-a-phase-2b-randomized-blinded-placebo-controlled/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dual-neutralization-of-il-17a-and-il-17f-with-bimekizumab-in-patients-with-active-ankylosing-spondylitis-48-week-efficacy-and-safety-results-from-a-phase-2b-randomized-blinded-placebo-controlled/
