Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: ABT-122 is a dual variable domain immunoglobulin (DVD-Ig™) that targets human tumor necrosis factor-α (TNF-α) and interleukin-17A (IL-17A). The object was to investigate the long-term safety and maintenance of efficacy of dual cytokine inhibition with ABT-122 for 24 weeks following the completion of a 12-week double-blind study.
Methods: The 24-week open-label extension (OLE) enrolled 158 patients (pts) receiving background methotrexate (MTX). All received ABT-122 120 mg subcutaneously every other week (eow) with options for 1 extra 120-mg dose (for inadequate efficacy) or downtitration to 60 mg eow (for safety reasons). Safety assessments included adverse events (AEs) and laboratory parameters. Maintenance of efficacy at week 24 of the OLE was evaluated with ACR 20/50/70 responses and low disease activity and clinical remission defined by 28-joint Disease Activity Score using high-sensitivity C-reactive protein (DAS28[hsCRP]) or Clinical Disease Activity Index (CDAI). Analyses using nonresponder imputation (NRI) for missing data are presented.
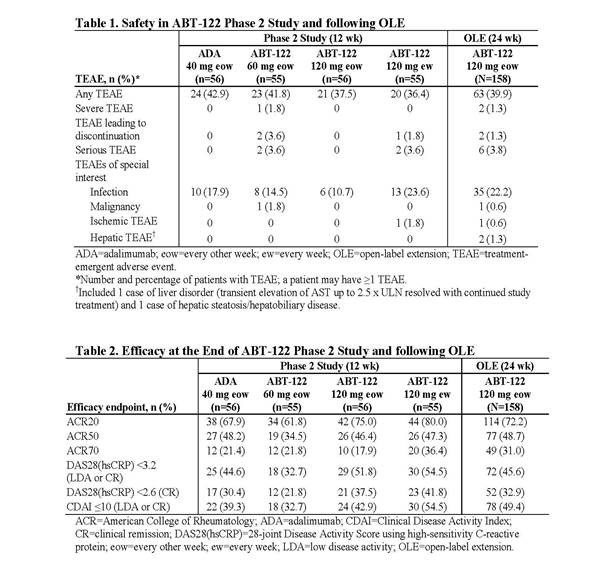
Results: The AE frequency appeared to be unchanged from the double-blind study and in line with that seen with the adalimumab (ADA) control arm in the 12-week study. Most AEs were mild or moderate (Table 1). Serious AEs were reported for 6 pts and included menorrhagia/anemia, myocardial ischemia, humerus fracture, joint dislocation, enterocolitis, and inflammation of the wound/skin ulcer; all events were unrelated to treatment as assessed by investigator and reported in a single term. Upper respiratory tract infection (n=14) and nasopharyngitis (n=4) were the most frequently reported infections. There were 1 nonserious report of basal cell carcinoma and 2 nonserious reports of liver events. There were no opportunistic infections, systemic hypersensitivity reactions, severe injection site reactions, cardiac failures, hematologic AEs, demyelinating disorders, or clinically concerning laboratory abnormalities. ABT-122 demonstrated consistent efficacy over 36 weeks across endpoints (Table 2), including pts who switched from ADA to ABT-122. Two pts had a downtitration to ABT-122 60 mg eow starting at week 2 or 4. An extra 120-mg dose of ABT-122 was given in 5 pts. Overall ACR20/50/70 responses were 72.2%/48.7%/31.0% at week 24 of the OLE.
Conclusion: Dual cytokine inhibition of TNF-α and IL-17A with ABT-122 demonstrated good tolerability and maintenance of effect in the OLE when dosed up to 36 weeks in pts with rheumatoid arthritis receiving background MTX, including those switching from ADA to ABT-122.
To cite this abstract in AMA style:
Genovese MC, Weinblatt M, Mansikka HT, Peloso PM, Chen K, Li Y, Liu J, Padley RJ. Dual Cytokine Inhibition with ABT-122, a Tnf– and IL-17–Targeted Dual Variable Domain Immunoglobulin (DVD-Ig™): Results from a 24-Week Open-Label Extension Study in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/dual-cytokine-inhibition-with-abt-122-a-tnf-and-il-17-targeted-dual-variable-domain-immunoglobulin-dvd-ig-results-from-a-24-week-open-label-extension-study-in-patients-wi/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dual-cytokine-inhibition-with-abt-122-a-tnf-and-il-17-targeted-dual-variable-domain-immunoglobulin-dvd-ig-results-from-a-24-week-open-label-extension-study-in-patients-wi/