Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: To describe drug retention rate and reasons for discontinuation of biological disease modifying anti-rheumatic drugs ( bDMARD) and novel small molecules in patients from the Singapore National Biologics Register (SNBR).
Methods: The SNBR is a prospective cohort started in 2016 in collaboration between the Rheumatology departments of National University Hospital, Tan Tock Seng Hospital and Singapore General Hospital to collect data about the epidemiology, disease activity, adverse effects and the development of comorbidities in patients treated with biologics and small molecules in patients with rheumatoid arthritis (RA) and spondyloarthropathy (SpA).
Patients with physician-diagnosed RA and SpA (axial (AS), peripheral and psoriatic arthropathy (PsA)) who were newly started on a bDMARD or small molecule, were recruited into this multi-centre, inception cohort. Data was collected via face-to face, telephone consultations and review of medical records at baseline, 6, 12, 18 and 24 months. Kaplan-Meier analysis for survival on drug by clinical diagnosis, income, educational level and financial assistance was performed.
Results: 126 patients had completed 2-years follow-up and were included in our analysis. 67.5% were female and 32.5% were male. 118 were initiated on bDMARDS and 8 on small molecules at the baseline visit (48.4% RA, 22.2% AS, 21.4% PsA and 7.9% other SpA). Baseline disease activity scores (mean) of DAS28-ESR in RA was 4.95 (SD 4.64-5.26) and in PsA was 3.97 (SD 3.55-4.39) while the mean BASDAI in AS was 4.38 (SD 3.56 -5.21) and in other SpA 5.59 (SD 4.1-7.08). At the end of 24 months, Kaplan-Meier estimated retention rate of the original bDMARD/small molecule was 41.1% (SD 33.0% -51.1%).
Reasons for discontinuation of these treatments are demonstrated in figure 1. The commonest reason for discontinuation was inefficacy of the treatment (30% in patients on anti-TNF agents and 38% on other biologics and novel small molecules respectively).
The survival on drug was higher in patients with axial spondyloarthropathy (AS) compared to those with peripheral SpA, PsA and RA (Figure 2). Educational level, funding source and income group did not significantly influence retention rate of these medications.
Conclusion: In our cohort at the end of a 2 year follow up, fewer than half of our patients on high cost bDMARDs/small molecules remained on their original bDMARD/small molecule. The retention rate of these medications was influenced by clinical diagnosis and inefficacy of the treatment was the main reason for discontinuation.
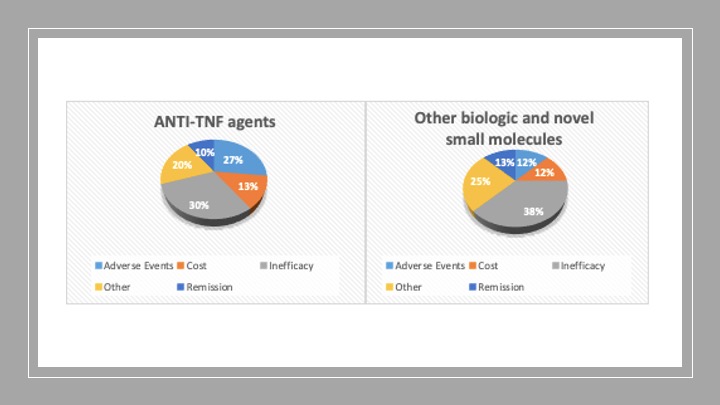 Figure 1: Reasons for discontinuation of biologic/novel small molecules
Figure 1: Reasons for discontinuation of biologic/novel small molecules
 Kaplan-Meier estimated drug retention rate by (clockwise from top left): underlying diagnosis (logrank p 0.0183), education (logrank p 0.1031), income group ( logrank 0.2748) and financial assistance ( logrank p 0.211).
Kaplan-Meier estimated drug retention rate by (clockwise from top left): underlying diagnosis (logrank p 0.0183), education (logrank p 0.1031), income group ( logrank 0.2748) and financial assistance ( logrank p 0.211).
To cite this abstract in AMA style:
Koh L, Aw M, Dhanasekaran P, Lim Mui San R, Wong S, Chua X, Choy W, Lahiri M, Yeo S. Drug Retention and Discontinuation of Biological DMARDs and Novel Small Molecules: Data from the Singapore National Biologics Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/drug-retention-and-discontinuation-of-biological-dmards-and-novel-small-molecules-data-from-the-singapore-national-biologics-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-retention-and-discontinuation-of-biological-dmards-and-novel-small-molecules-data-from-the-singapore-national-biologics-registry/
