Session Information
Date: Monday, November 14, 2022
Title: RA – Treatment Poster IV
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Sustained remission has become an achievable treatment goal for many patients with RA, and drug-free remission has been proposed as a potential extended target for some of these patients. However, trials assessing outcomes following complete withdrawal of DMARD in long-term deep remission are limited. The primary objective of this study was to assess the effect of discontinuation of csDMARDs on the risk of flares in RA patients who had been in sustained clinical remission for at least two years, and who had already successfully tapered their csDMARDs to half-dose.
Methods: This randomized clinical trial is part two of the ARCTIC REWIND project studying tapering of csDMARDs. In the first part, patients in sustained remission ≥12 months were randomized to stable or half-dose therapy (Lillegraven et al, JAMA 2021). In the present study, those who remained flare-free for 12 months on half-dose treatment (and had thus been in remission ≥24 months altogether), were randomized 1:1 to discontinuation or continued half-dose csDMARDs. The primary endpoint was disease flare during one year, defined as a combination of DAS > 1.6, a change in DAS ≥ 0.6 and at least 2 swollen joints, or that both the physician and patient agreed that a clinically significant flare had occurred. The difference in flare risk between the treatment groups was estimated using a risk-difference estimator with stratification for study center. Radiographic joint damage was scored by van der Heijde modified Sharp score (progression: ≥1 unit change/year). Clinicaltrials.gov number NCT01881308.
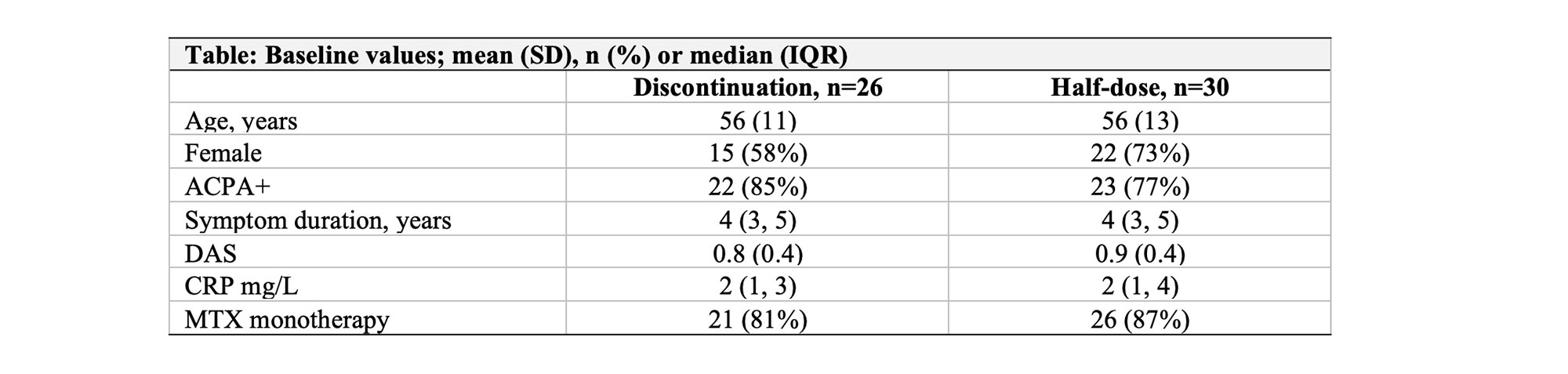
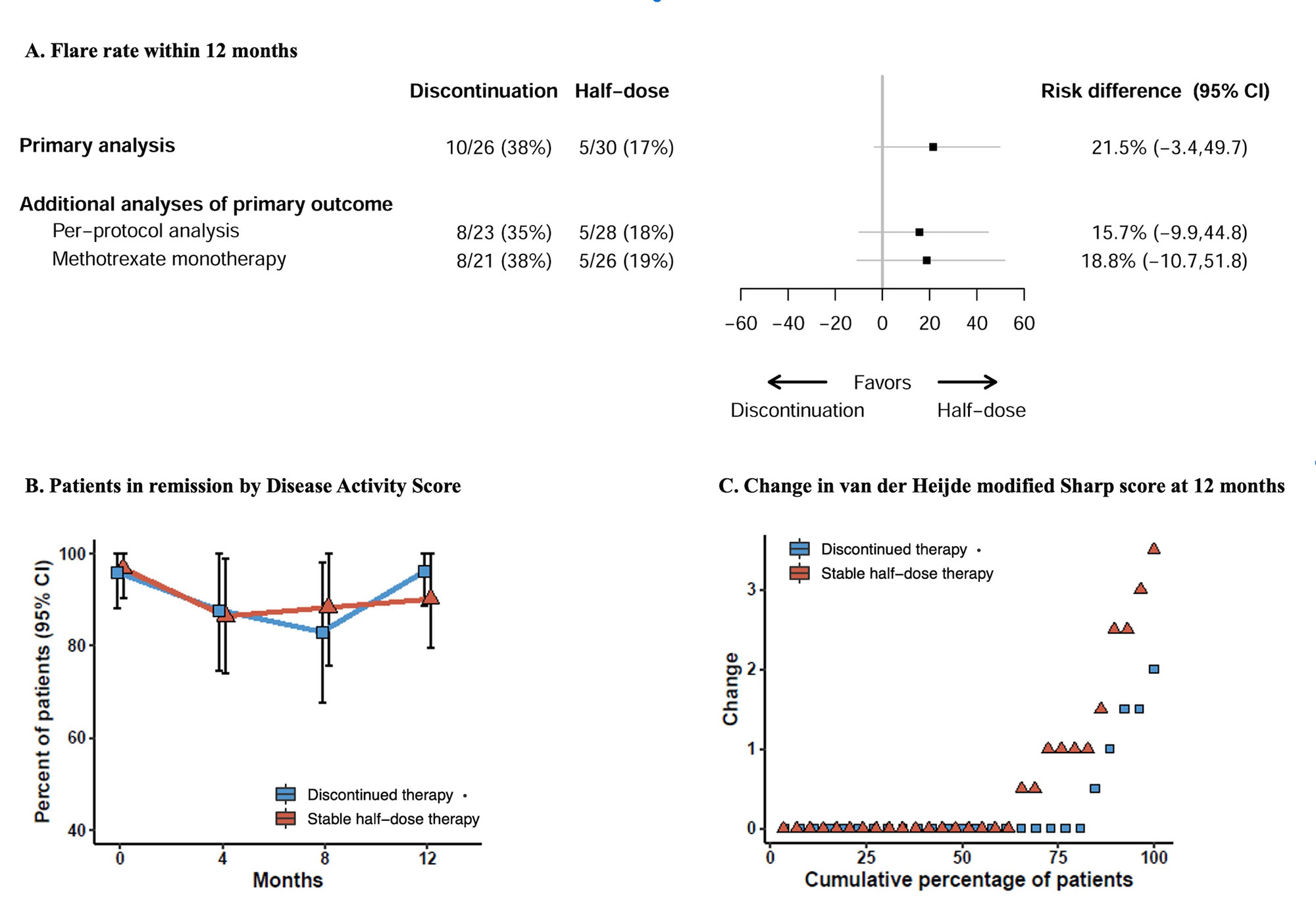
Results: 56 patients were randomized and attended at least one efficacy evaluation. Baseline characteristics were mostly well balanced (Table 1). Mean (SD) DAS in the discontinuation group was 0.8 (0.4) compared to 0.9 (0.4) in the stable half-dose group, mean baseline methotrexate dose in both groups 10 mg/week. In the primary analysis, 10/26 (38%) of patients discontinuing csDMARD experienced a flare during 12 months, compared to 5/30 (17%) in the stable half-dose group, risk difference (95% CI) 22% (-3% to 50%, Figure 1, p-value 0.13). In the discontinuation group, 10/10 (100%) adjusted DMARD medication following the flares, corresponding numbers in the half-dose group 4/5 (80%). There was no difference in the proportion of patients with absence of progression of radiographic joint damage; 84% of patients discontinuing therapy versus 69% on stable half-dose, risk difference (95% CI) 14% (-11% to 38%). After 12 months, 96% in the discontinuation group and 90% in the half-dose group were in DAS remission. The total number of adverse events was 22 in the discontinuation group and 26 in the half-dose group (one serious adverse event).
Conclusion: In RA patients who had sustained clinical remission for at least two years and successfully tapered csDMARDs to half-dose, a majority of patients who discontinued csDMARDs remained flare free for 12 months. The risk of disease flare was not significantly different compared to patients continuing stable half-dose csDMARDs, however the sample size was limited, and a numeric difference in flares were observed in favor of continuing half-dose treatment. The results provide a basis for shared decision making.
To cite this abstract in AMA style:
Lillegraven S, Sundlisæter N, Aga A, Sexton J, Olsen I, Fremstad H, Spada C, Madland T, Høili C, Bakland G, Lexberg Å, Hansen I, Hansen I, Haukeland H, Ljoså M, Moholt E, Uhlig T, Solomon D, van der Heijde D, Kvien T, Haavardsholm E. Drug Free Remission in Rheumatoid Arthritis: Data from the Randomized Controlled ARCTIC REWIND Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/drug-free-remission-in-rheumatoid-arthritis-data-from-the-randomized-controlled-arctic-rewind-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-free-remission-in-rheumatoid-arthritis-data-from-the-randomized-controlled-arctic-rewind-trial/