Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
The best long-term treatment strategy for rituximab has not been established. Retreatment at a fixed interval of 6 months maintains stable disease activity1 and half-dose is equally effective in first-cycle responders1. In first-cycle non- or moderate responders, responses may improve further after a second cycle at full dose2,3.
We used a strategy of fixed 6-monthly retreatment at half-dose following an initial full dose cycle in responders and non-responders, and looked for changes in clinical response.
Methods:
Patients received 2x1000mg rituximab at month 0 (C1), 2x500mg rituximab at month 6 (C2) and 2x500mg at month 12 (C3) regardless of C1 response. All patients were positive for RF and/or anti-CCP. 18/41 were taking concomitant MTX and 9/41 other DMARDs. 2 patients were taking concomitant oral prednisolone. DAS28 was measured at baseline and at 3-6 months after each cycle and compared to baseline of the first cycle.
Results:
To date, 41 patients received C1, 34 C2, 17 C3 and 14 C4 with outcome data. For all patients, mean(SD) DAS28 at baseline and after C1, C2 and C3 were 6.15(0.74), 4.14(1.10), 3.82(1.14) and 3.13(1.06) respectively. EULAR Non/Moderate/Good responses were achieved by 5, 28 and 8/41 patients (12/68/20%) in C1; 5, 18 and 11/34 patients (15/53/33%) in C2; 1, 6, 10/17 patients (6/35/59%) in C3; and 1, 3 and 10/14 patients in C4 (7/21/71%). 3/5 patients with Non response in C1 responded to C2.
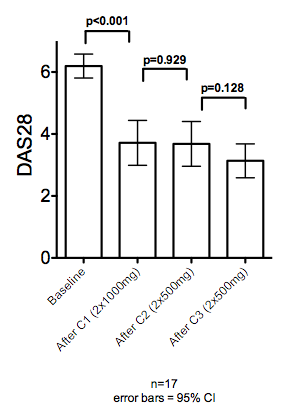
Proportions of patients with a change in EULAR response between C1-C2 and C2-C3 was compared for the 17 patients who received 3 cycles. For C1-C2: 53% patients maintained the same EULAR response, 18% improved and 29% worsened. For C2-C3, 47% maintained the same response, 41% improved and 12% worsened. DAS28 for these patients is shown in Figure 1. There was no significant difference between C1 and C2 and a trend to reduction in DAS28 after C3 (p=0.128, paired t-test).
Conclusion:
Some C1 non-responders responded to retreatment with half-dose at 6 months, but response rate across all patients was similar. Incremental improvements in C1 non- or moderate responders were seen more frequently after a second half-dose retreatment. This suggests that dose reduction may delay achievement of optimal responses in C1 non- or moderate responders, and these patients should have 2 full-dose cycles before reducing doses. Future work will analyse B cell depletion in this cohort.
References
1. Dougados et al. Ann Rheum Dis 2012;71(Suppl3):182
2. Rubbert-Roth et al. Rheumatology (2010) 49(9):1683-93
3. Vital et al. A&R (2010) 62(5):1273-9
Figure 1. DAS28 at baseline and after C1(2x1000mg) and C2 and C3 (2x500mg).
Disclosure:
M. I. Sharif,
None;
S. Das,
None;
P. Emery,
Roche Pharmaceuticals,
5,
Roche Pharmaceuticals,
2;
H. MacIver,
None;
W. Shingler,
None;
P. S. Helliwell,
None;
K. Sokoll,
None;
E. M. Vital,
Roche Pharmaceuticals,
2,
Roche Pharmaceuticals,
8.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dose-reduction-in-rituximab-retreatment-may-delay-achievement-of-optimal-responses/