Session Information
Date: Friday, November 6, 2020
Title: Patient Outcomes, Preferences, & Attitudes I: COVID-19 (0464–0468)
Session Type: Abstract Session
Session Time: 5:00PM-5:50PM
Background/Purpose: Due to concerns about underlying immune dysregulation and immunosuppression, patients with systemic rheumatic diseases living in COVID-19 “hot spots” may have modified their immunomodulatory medications during the peak of the pandemic in an effort to minimize risk and severity of infection. We aimed to assess the degree of immunomodulatory medication modification at a large specialty hospital.
Methods: We emailed a secure web-based survey to 26,045 patients aged ≥18 years evaluated at least once by a rheumatologist between April 1, 2018-April 21, 2020 at a tertiary care academic center in New York City. Patients were invited to complete the survey by email or phone between April 24, 2020 and May 26, 2020. Detailed information was collected, including potential COVID-19 exposure, symptoms, and outcomes, as well as rheumatic disease history and medications. Patients were asked to report any immunomodulatory medication use in the previous six months and to indicate whether they increased, decreased or discontinued the medication during the COVID-19 pandemic, as well as reasons for reduction or discontinuation.
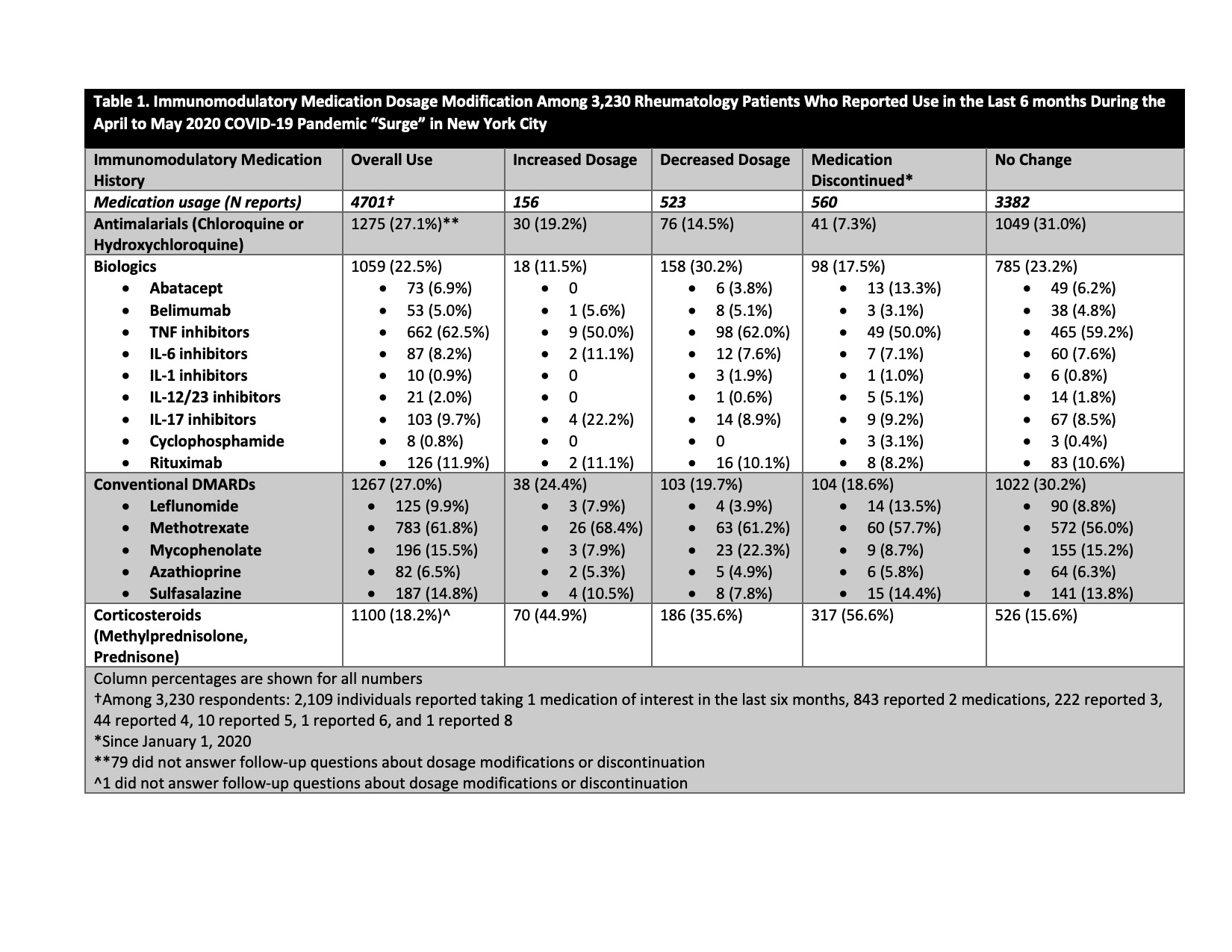
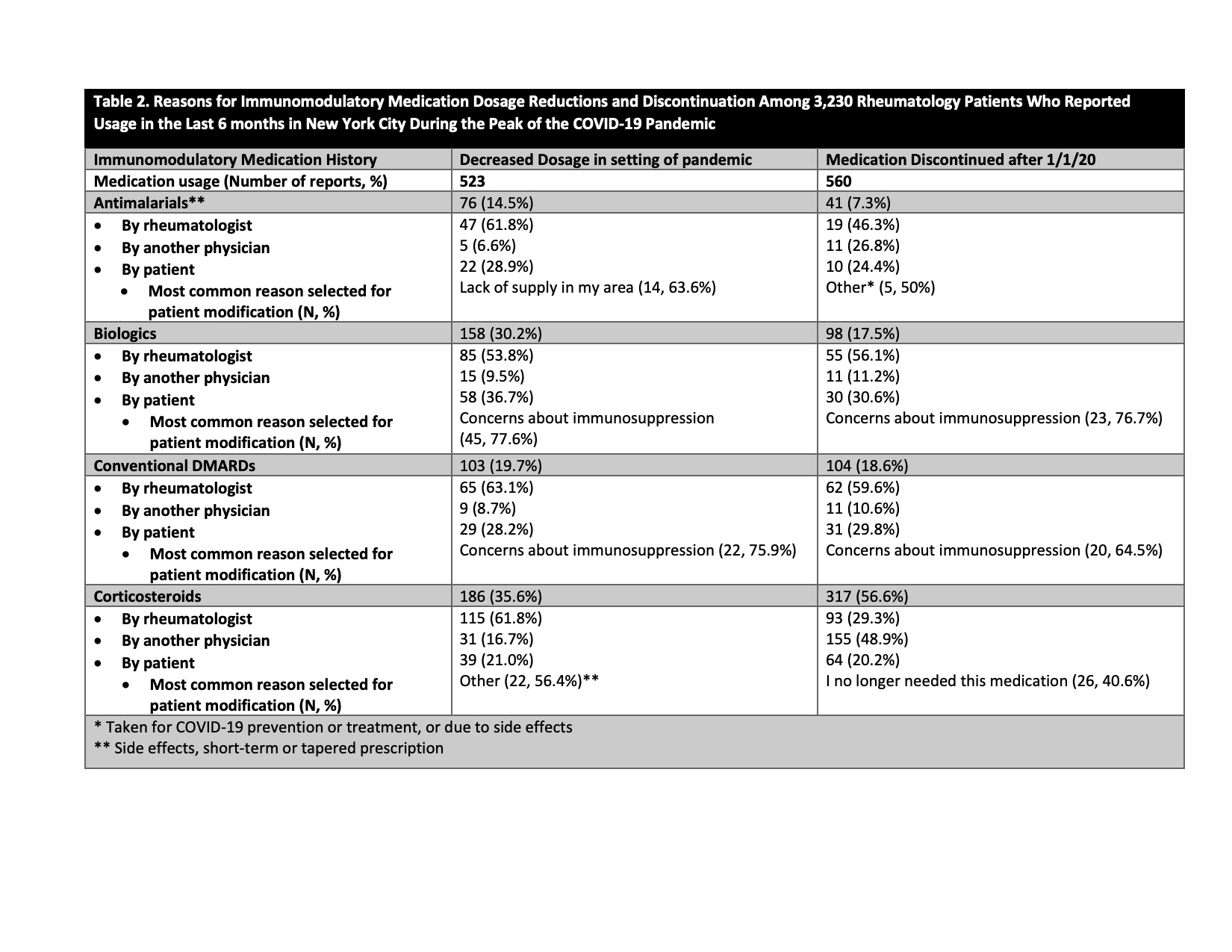
Results: 6,908/26,045 patients (26.5%) responded to the survey. Mean age was 59.6±15.6 years, 76.3% were female, 86.7% were white, and 7.4% were Hispanic/Latinx. 3,230 patients (46.8% of respondents) reported any use of antimalarial drugs, biologic therapies, conventional DMARDs), and/or corticosteroids in the previous six months. 1275/6,908 (18.5%) patients used antimalarials, 1033 (15.0%) used biologic therapies, 1208 (17.5%) used conventional DMARDs, and 1100 (15.9%) used corticosteroids. Among 3,230 patients, there were 4,701 individual reports of immunomodulatory drug use (2,109 used 1 medication, 843 used 2 medications, 278 used ≥2 medications). Medication dosages were increased 156 times (3.3% of reports), decreased 523 times (11.1%), unchanged 3,382 times (71.9%), and discontinued 560 times (11.9%) (Table 1). We collected only one modification per medication during the study time period. Among dosage reduction reports, 35.6% were corticosteroids, 30.2%r biologics, 19.7% conventional DMARDs, and 14.5% antimalarials. Medication discontinuation was highest for corticosteroids (56.6%), less for conventional DMARDs (18.6%) and biologics (17.5%), and lowest for antimalarials (7.3%). 19.2% of reported increases in medication doses were for antimalarials. Medication reductions were advised >50% of the time by a physician, whereas up to a third of discontinuations were self-directed (Table 2).
Conclusion: During the peak of the COVID-19 pandemic, nearly a quarter of immunomodulatory drugs used by surveyed rheumatology patients at a large, tertiary care, specialty center in NYC were reduced or discontinued. These findings provide insight into real-world medication management of patients with rheumatic disease during an infectious disease pandemic. Understanding patient and physician behavior during this public health crisis will help guide planning for a second Sars-CoV-2 spike or future pandemic. Longitudinal studies will evaluate the impact of changes to routine medications on disease activity among these patients.
To cite this abstract in AMA style:
Frey M, Vitone G, Feldman C, Lally L, Bass A, Salmon J, Crow M, Lockshin M, Bykerk V, Mandl L, Barbhaiya M. Dosage Modification of Immunomodulatory Medications by Rheumatology Patients in New York City During the Peak of the COVID-19 Pandemic [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/dosage-modification-of-immunomodulatory-medications-by-rheumatology-patients-in-new-york-city-during-the-peak-of-the-covid-19-pandemic/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dosage-modification-of-immunomodulatory-medications-by-rheumatology-patients-in-new-york-city-during-the-peak-of-the-covid-19-pandemic/