Session Information
Date: Sunday, November 10, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Patient Reported Outcomes
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) patients are often not in remission due to patient global assessment of disease activity (PtGA) which is included in the formula of all disease activity indices. Given that PtGA may reflect pain or fatigue, the aim of this analysis was to assess the relative timing and perhaps lag of patient-reported outcomes (PRO) after remission is obtained as measured by clinical disease activity index (CDAI) or swollen joint count (SJC28) in a large observational database of RA patients followed in routine clinical care.
Methods: RA patients enrolled in the Ontario Best Practices Research Initiative (OBRI) registry that were not in low disease state at baseline based on the CDAI, SJC28, PtGA, pain and fatigue criteria below, and had at least six months of follow-up, were included in the analysis. Low disease state was defined as CDAI≤10, SJC28≤2, PtGA≤2cm, pain score≤2cm, or fatigue score≤2cm. Remission was defined as CDAI≤2.8, SJC28≤1, PtGA≤1cm, pain score≤1cm, or fatigue score≤1cm. Kaplan-Meier survival analysis was used to assess the time to first low disease state / remission based on each definition.
Results: A total of 986 patients were included with a mean (SD) age and disease duration of 57.4 (12.9) years and 8.3 (9.9) years, respectively, and mostly women (80.0%). Mean (SD) of CDAI, SJC28, PtGA, pain, and fatigue at enrolment was 29.8 (11.7), 8.3 (4.6), 6.4 (1.9), 6.6 (1.9), and 6.7 (2.0), respectively.
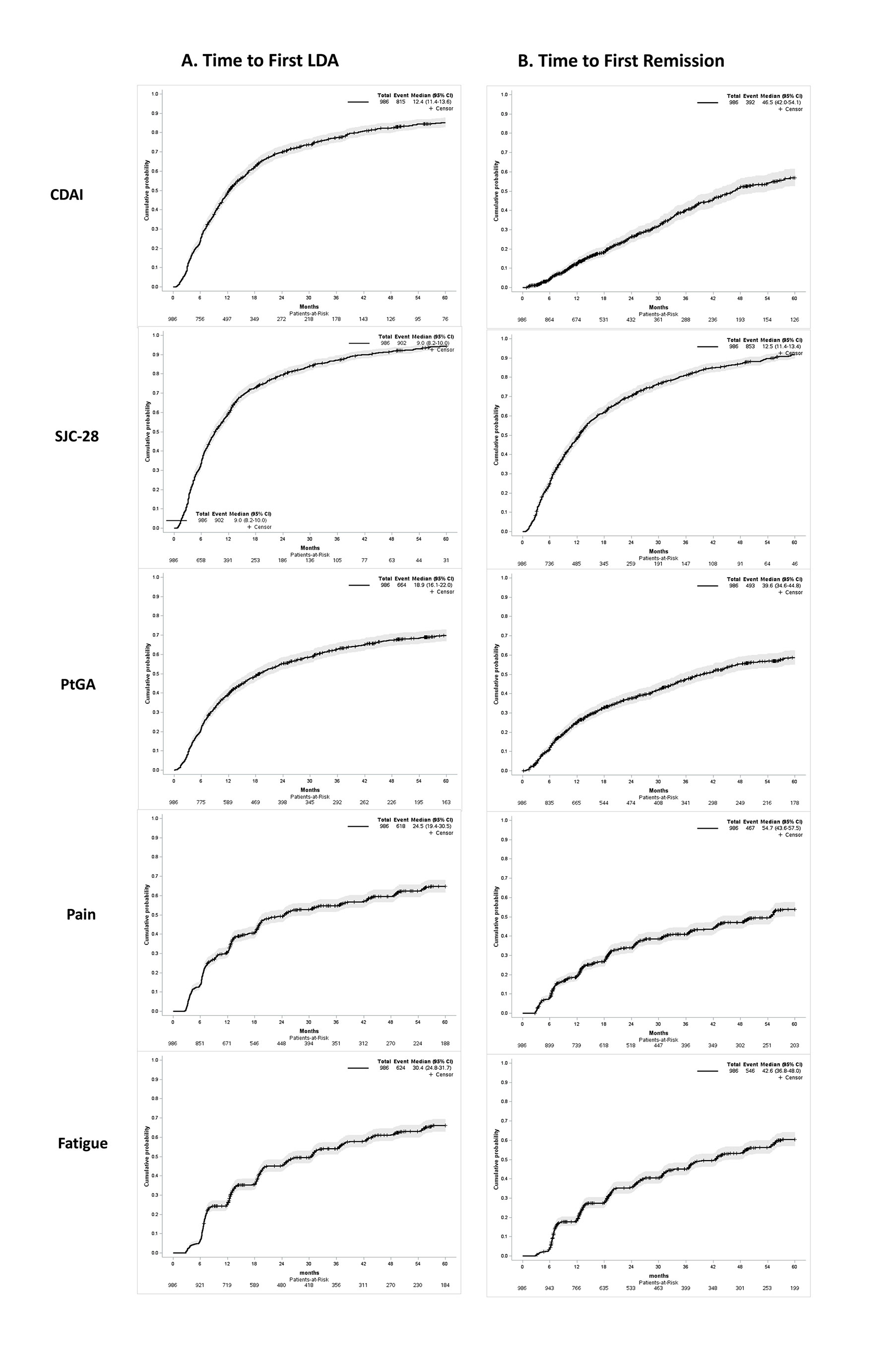
Figure 1 shows the time to first low disease state and time to first remission based on different definitions. The median (95%CI) time in months to CDAI≤10 was 12.4 (11.4-13.6), SJC28≤2 was 9 (8.2-10), PtGA≤2cm was 18.9 (16.1-22), pain≤2cm was 24.5 (19.4-30.5), and fatigue≤2cm was 30.4 (24.8-31.7). For remission, the median (95%CI) time to CDAI≤2.8 was 46.5 (42-54.1), SJC28≤1 was 12.5 (11.4-13.4), PtGA≤1cm was 39.6 (34.6-44.8), pain≤1cm was 54.7 (43.6-57.5), and fatigue≤1cm was 42.6 (36.8-48).
Conclusion: Time to achieving low disease state or remission based on PROs is considerably longer compared to swollen joint count which may have significant impact on the time to CDAI low disease activity and remission. Remission and low disease activity composite scores, and PROs lag behind SJCs. Careful interpretation of PROs and composite scores could impact management including prevention of overtreatment and unnecessary switching of DMARDs.
To cite this abstract in AMA style:
Pope J, Rampakakis E, Movahedi M, Cesta A, Sampalis J, Bombardier C. Does Improvement in Patient Pain and Fatigue Lag Behind Clinical Remission in Rheumatoid Arthritis Patients? Data from a Rheumatoid Arthritis Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/does-improvement-in-patient-pain-and-fatigue-lag-behind-clinical-remission-in-rheumatoid-arthritis-patients-data-from-a-rheumatoid-arthritis-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/does-improvement-in-patient-pain-and-fatigue-lag-behind-clinical-remission-in-rheumatoid-arthritis-patients-data-from-a-rheumatoid-arthritis-registry/