Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient reported outcomes (PROs) are important in the treatment evaluation of patients with spondyloarthritis, including axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA). For secukinumab, limited real world data on PROs is available – and no comparison between axSpA and PsA patients has been performed. In European patients with axSpA and PsA who initiated secukinumab, we aimed to determine: 1) the proportion of patients achieving 6-, 12- and 24-month pain, fatigue, patient global assessment (PGA) and health assessment questionnaire (HAQ) remission, and 2) the 24-month secukinumab retention rate.
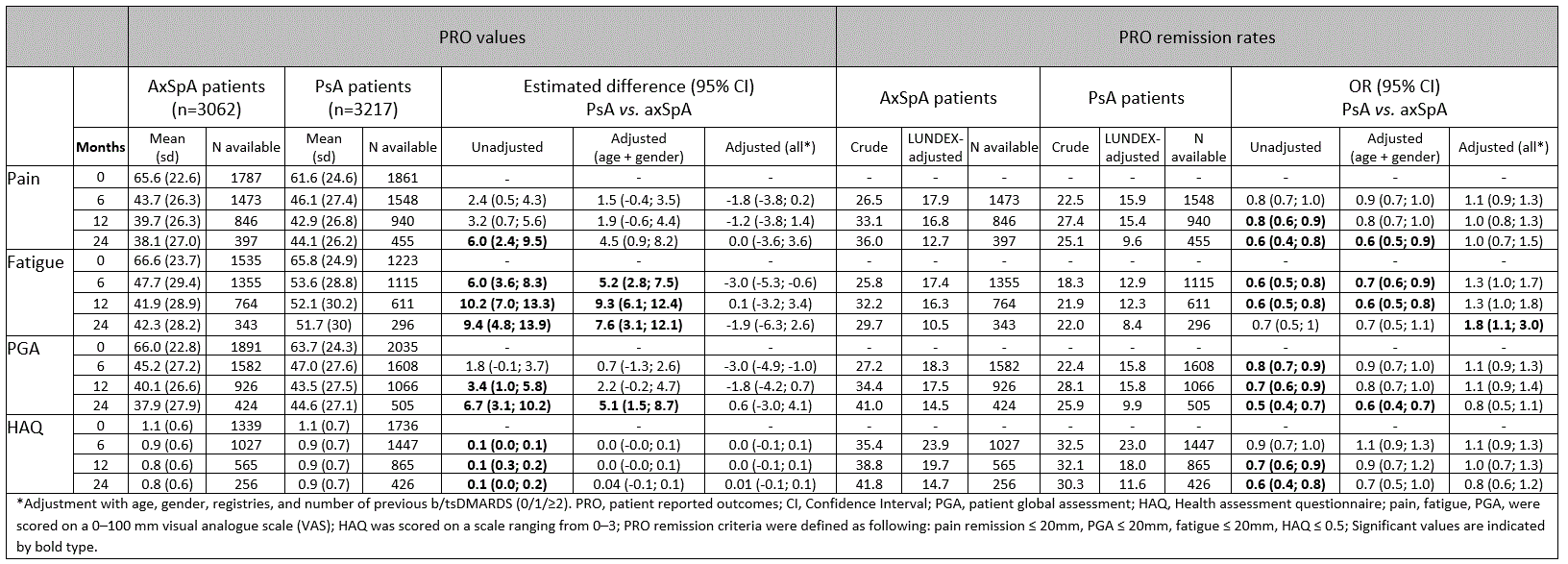
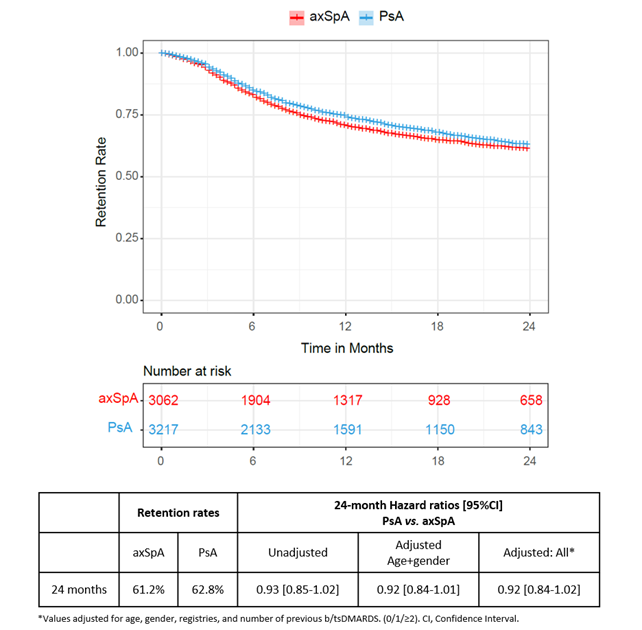
Methods: The study was conducted within the European Spondyloarthritis Research Collaboration Network (EuroSpA)(1). From 16 European registries, patients with axSpA or PsA who initiated secukinumab between 2015 and 2021 were included. Based on the ASAS working group definition of partial remission in axSpA(2), we applied the following definitions of PRO remission: pain≤20, PGA≤20, fatigue≤20 (all on visual analogue scales 0-100 mm) and HAQ≤0.5 for both axSpA and PsA patients, to make comparisons feasible.PRO remission rates were calculated as crude and adjusted for secukinumab adherence (LUNDEX). Comparisons of remission rates were performed with univariable and multivariable (baseline covariates: age, gender, registry, and number of previous b/tsDMARDs)logistic regression analyses. Kaplan-Meier with log-rank test and Cox regression analyses were performed to assess and compare the 24-month secukinumab retention rate between axSpA and PsA patients.
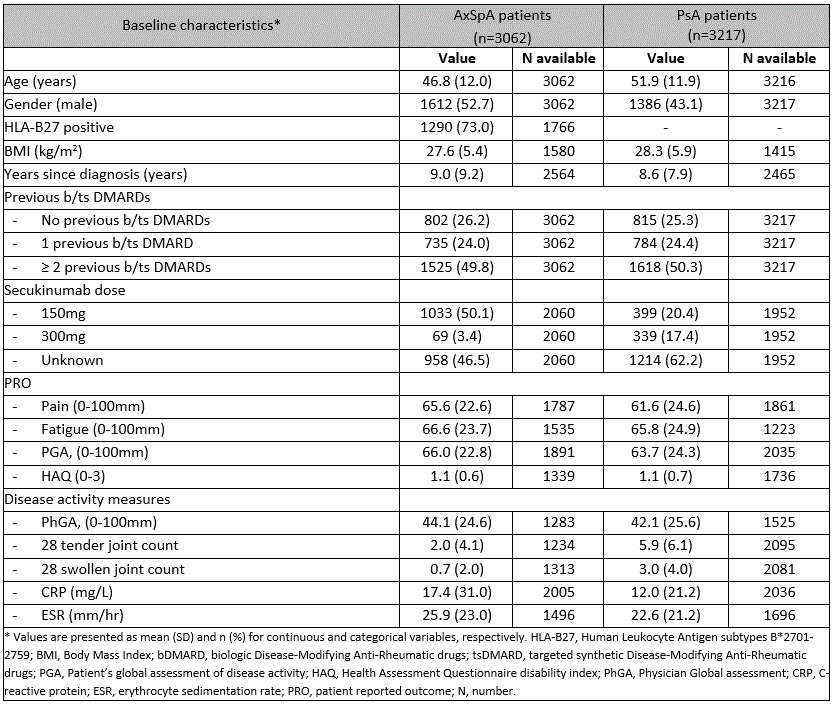
Results: We included 3062 axSpA patients and 3217 PsA patients initiating secukinumab in routine care. At secukinumab treatment start (baseline), axSpA patients had a mean (SD) age of 46.8 (12.0) years, with 52.7% being male. PsA patients had a mean age (SD) of 51.9 (11.9) years and were predominantly female (56.9%). No clinically relevant differences in disease duration, physician global assessment (PhGA), number of previous b/tsDMARDs or PROs values were found at baseline (Table 1). The reductions in pain, fatigue, and PGA values from baseline to 24-month follow-up were greater in axSpA patients compared to PsA patients. While crude PRO remission rates (pain, fatigue, PGA and HAQ) were higher for axSpA than for PsA patients at all timepoints, LUNDEX-adjusted remission rates were similar (Table 2). After correction for multiple confounders no difference was found between the groups, except for a higher remission rate for fatigue in PsA at 24 months (OR= 1.8 [95% CI 1.1-3.0]) (Table 2). The 24-month retention rates were similar in axSpA and PsA (61.2% vs. 62.8%, HR= 0.92 [95% CI 0.84-1.02], p=0.14 in fully adjusted analyses) (Figure 1).
Conclusion: Our study supports the real-world effectiveness of secukinumab in both axSpA and PsA, as measured by PROs, and suggests that PROs (including pain, PGA and HAQ) and 24-month retention rate after secukinumab initiation are similar in axSpA and PsA patients. Further analyses, aiming to evaluate long-term effectiveness of axSpA and PsA are needed.
References
1. Anon. https://eurospa.eu/.
2. Anderson JJ et al. Arthritis and Rheumatism 2001;44:1876–1886.
To cite this abstract in AMA style:
Pons M, Horskjær Rasmussen S, Nysom Christiansen S, Michelsen B, Glintborg B, Gudbjornsson B, Grondal G, Vencovsky J, Loft A, Rotar Z, Perdan Pirkmajer K, Nissen M, Moeller B, Macfarlane G, Jones G, Iannone F, Caporali R, Laas K, Vorobjov S, Di Giuseppe D, Nihan Coskun B, Yagız B, Provan S, Fagerli K, Castrejon I, Otero-Valera L, van de Sande M, van der Horst-Bruinsma I, Nordstrom D, Kuusalo L, Vieira-Sousa E, Bernardes M, Olofsson T, Baranová J, Hetland M, Østergaard M, Ørnbjerg L. Do Long-term Patient-reported Outcomes Improve Similarly in Psoriatic Arthritis and Axial Spondyloarthritis Patients Treated with Secukinumab? Results from the EuroSpA Collaboration [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/do-long-term-patient-reported-outcomes-improve-similarly-in-psoriatic-arthritis-and-axial-spondyloarthritis-patients-treated-with-secukinumab-results-from-the-eurospa-collaboration/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/do-long-term-patient-reported-outcomes-improve-similarly-in-psoriatic-arthritis-and-axial-spondyloarthritis-patients-treated-with-secukinumab-results-from-the-eurospa-collaboration/