Session Information
Date: Wednesday, November 13, 2019
Title: 6W021: RA – Treatments V: Switching & Tapering RA Medications (2906–2911)
Session Type: ACR Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: In rheumatoid arthritis (RA) disease outcomes have improved enormously in the last decades. Due to early initiation of therapy, a treat-to-target approach and a growing arsenal of disease-modifying antirheumatic drugs (DMARDs) and biologicals, RA patients are in sustained remission more often. This raises the subsequent questions whether treatment should be continued, tapered or stopped. Although DMARD free remission (DFR) is nowadays achievable for increasing numbers of RA patients, the optimal tapering approach still needs to be defined. In this study, we want to evaluate the 2-year clinical effectiveness of two gradual tapering strategies, namely tapering DMARDs first followed by the TNF-inhibitor, or vice versa.
Methods: In this multicenter single-blinded randomised controlled trial RA patients with controlled disease for at least 3 consecutive months, defined as a DAS≤2.4 and a swollen joint count (SJC) ≤1, which was achieved with csDMARDs and a TNF-inhibitor were included. Eligible patients were randomised into gradual tapering csDMARDs followed by the TNF blocker or vice versa. Medication was tapered in three steps over the course of 6 months. After 12 months, the other DMARD was tapered. Gradual tapering was done by cutting the dosage into half, a quarter and thereafter it was stopped. The primary outcome for the clinical effectiveness was the number of patients with a disease flare, defined as DAS44 >2.4 and/or SJC >1. Secondary outcomes were DFR, disease activity, quality of life and functional ability. Outcomes were calculated in an intention-to-treat analysis, using all available data.
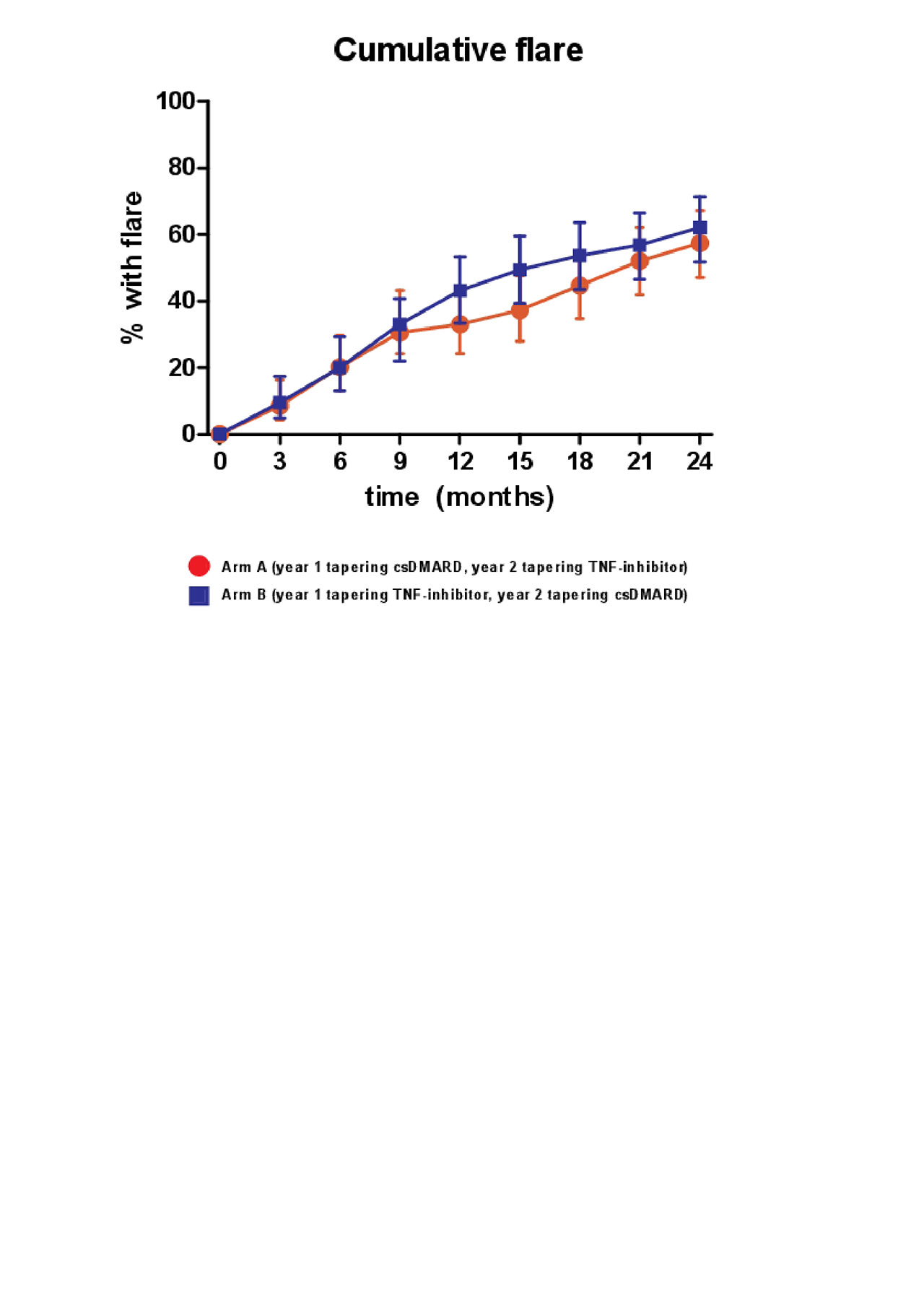
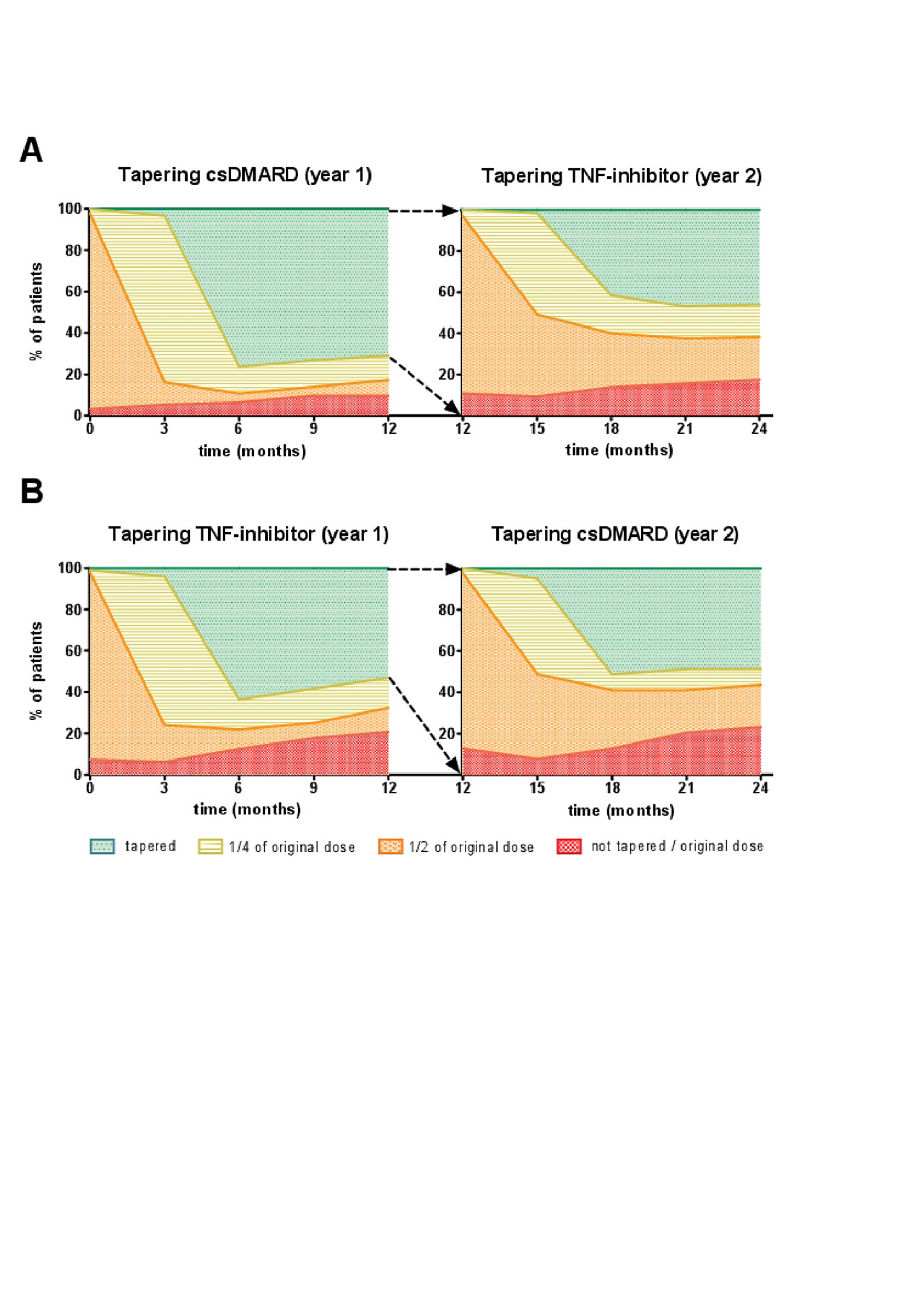
Results: A total of 189 patients were randomly assigned to tapering csDMARDs first (arm A) or tapering TNF-inhibitor first (arm B). The cumulative flare rate after 24 months in A was 61% (95% CI, 50% – 69%) and in B 62% (95% CI, 50%-70%) (p=0.35)(figure 1). In the second year the cumulative flare rate differs the most between the two groups between T12 and T18 (figure 1). Figure 2 shows an overview of how gradual tapering took place in the TARA study, and how many patients were at a certain point of tapering at the separate time-points (figure 2). This was indicated for both tapering arms (A and B), and for the first and second year (figure 2). In arm A there were significantly more patients able to taper their medication (n=30), and more patients reached DFR (n=15) (table 1). However, mean DAS, mean HAQ, and mean EQ5D over time, and after 2 years, did not differ between both tapering arms (table 1).
Conclusion: The order in which tapering and stopping medication was performed was not superior to each other based on flare rates, DAS and HAQ. However, patients that first tapered csDMARD reached DFR more often and more patients were able to taper their medication.
To cite this abstract in AMA style:
van Mulligen E, Weel A, Kuijper M, Hazes M, van der Helm-van Mil A, de Jong P. DMARD-free Remission in Established Rheumatoid Arthritis: 2 Year Results of the TARA Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/dmard-free-remission-in-established-rheumatoid-arthritis-2-year-results-of-the-tara-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dmard-free-remission-in-established-rheumatoid-arthritis-2-year-results-of-the-tara-trial/