Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Connective tissue diseases (CTDs) are a heterogeneous group of diseases with overlapping clinical features and shared immunopathology. In routine practice, a clinician diagnosis is often made without direct reference to classification criteria however meeting classification criteria is important when recruiting to studies and trials and in accessing medicines. We aimed to assess whether patients clinically diagnosed with a CTD met the relevant classification criteria and describe their clinical manifestations and immunological profile. Secondly, we applied accepted classification criteria to each individual patient.
Methods: Adult patients were recruited with one or more clinical feature suggestive of a CTD and one or more antibody within the antinuclear spectrum. Patients were initially grouped according to clinician diagnosis and we described their clinical features and immunological profile. Patients were then reviewed and classified, where appropriate, using SLICC SLE 2012 criteria, ACR/EULAR 2016 Sjögren’s syndrome criteria, ACR/EULAR 2013 Systemic Sclerosis (SSc) criteria, the Bohan and Peter 1975 Myositis criteria, Rheumatoid Arthritis (RA) 2010 ACR/ EULAR criteria and Antiphospholipid syndrome (APS) -Modified Sydney criteria, 2006.
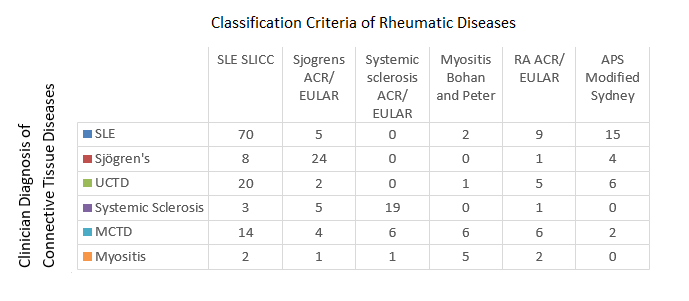
Results: We recruited 249 patients from May 2014-April 2019. Of these, 227 (91.2%) were female with a mean (SD) age of 48.5 (13.0) years. By clinician diagnosis, 86 (34.5%) had SLE, 35 (14.1%) had Sjögren’s syndrome, 58 (23.3%) had UCTD, 26 (10.4%) had SSc, 32 (12.9%) had Mixed Connective Tissue Disease (MCTD) and 12 (4.8%) had myositis. Disease duration was shortest in patients with UCTD and myositis (median 3 years), and longest in SLE (median 10 years).
Patients from each clinician diagnosed disease group met SLICC SLE criteria including 20 (34.5%) UCTD and 14 (43.8%) MCTD patients (Fig 1). SLICC/SLE mucocutaneous criteria (including ulcers, alopecia and cutaneous lesions) and arthritis were prevalent across all diseases, affecting 83 (96.5%) SLE patients, 20 (57.1%) of Sjögren’s syndrome, 45 (77.6%) UCTD, 12 (46.2%) SSc, 29 (90.6%) MCTD and 6 (50%) myositis patients. In contrast, deep organ involvement (such as renal, neurological and serositis) satisfying SLICC SLE criteria, were mostly observed in SLE patients.
Anti-dsDNA antibodies were found in 22.9% of Sjögren’s patients and 19% of UCTD patients (Fig 2). Anti-centromere antibodies had a high specificity for SSc. In contrast, other auto-antibodies were prevalent across a number of CTDs, in particular rheumatoid factor and low complement were observed in all conditions.
Conclusion: Within this CTD cohort, patients met classification criteria for CTDs other than the diagnosis given by the treating clinician and the autoantibody spectrum observed showed significant overlaps across diagnostic and classification subsets. Of note a high proportion of UCTD and MCTD patients fulfil criteria for other CTDs. Our study suggests that CTD patients are likely to have overlapping clinical and immunological phenotypes and they need to be continually reassessed for new or evolving clinical features.
To cite this abstract in AMA style:
Dyball S, Reynolds J, Chinoy H, Briggs T, Haque S, McCarthy E, Bruce E, Herrick A, Bruce I, Parker B. Disentangling Connective Tissue Diseases: Overlaps and Disparities in Clinical Diagnosis, Classification Criteria and Autoantibodies – Results from the Lupus Extended Autoimmune Phenotype Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/disentangling-connective-tissue-diseases-overlaps-and-disparities-in-clinical-diagnosis-classification-criteria-and-autoantibodies-results-from-the-lupus-extended-autoimmune-phenotype-stud/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disentangling-connective-tissue-diseases-overlaps-and-disparities-in-clinical-diagnosis-classification-criteria-and-autoantibodies-results-from-the-lupus-extended-autoimmune-phenotype-stud/