Session Information
Date: Tuesday, November 9, 2021
Title: Pediatric Rheumatology – Clinical Poster III: Miscellaneous Rheumatic Disease (1614–1644)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Patients with chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperatures /proteasome-associated autoinflammatory syndrome (CANDLE/PRAAS) respond to treatment with baricitinib but require higher exposure than doses used to treat patients with rheumatoid arthritis (1, 2). After clinical observations of disease flares associated with baricitinib dose reductions due to a possible adverse event, we systematically assessed the effect of baricitinib dose reductions on the CANDLE/PRAAS disease activity and leveraged these observations for developing and validating “disease flare criteria” that can be used to quantify disease activity and in clinical study design.
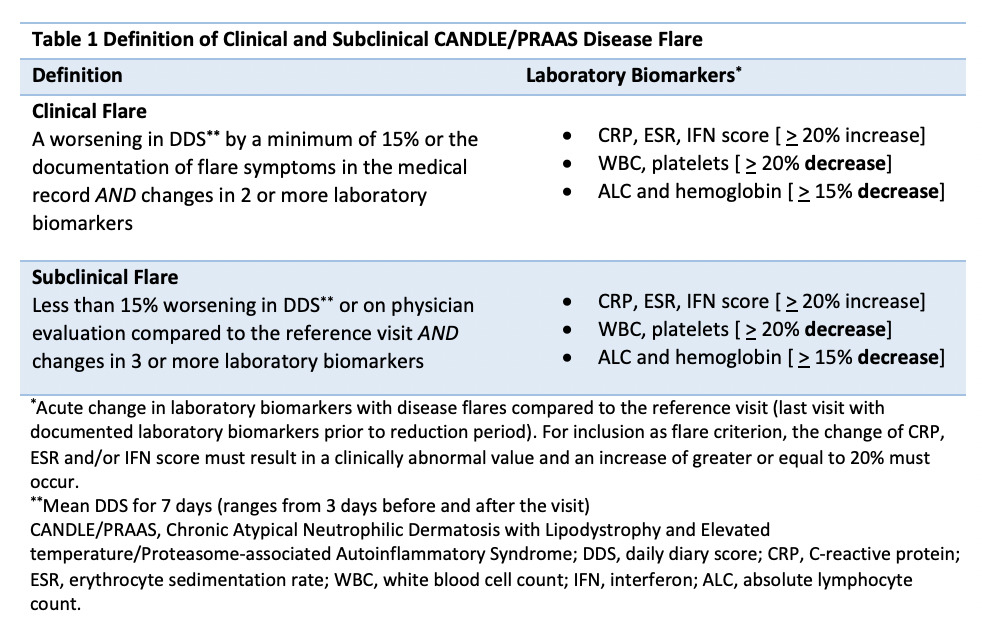
Methods: Between October 2011 and August 2018, 10 genetically confirmed CANDLE patients were enrolled in an institutional review board approved open label expanded access program (NCT01724580) and treated with baricitinib. Patients or their parents provided written informed consent. We retrospectively identified all instances of dose reductions and assessed clinical symptoms recorded on a daily diary, laboratory markers of inflammation and an interferon (IFN) score. We observed and characterized clinical symptoms and laboratory changes during disease flare periods and developed “flare criteria” that captured clinically meaningful changes (table 1). Time to flare was evaluated using Kaplan-Meier analysis and the Cox proportional hazards model. To validate the flare criteria, we compared the baricitinib dose reduction associated flare rate during the flare period (post dose reduction) with the flare rate during a stable period (on optimal baricitinib dose) for patients who fulfilled flare criteria using a two-sided chi-squared test of homogeneity.
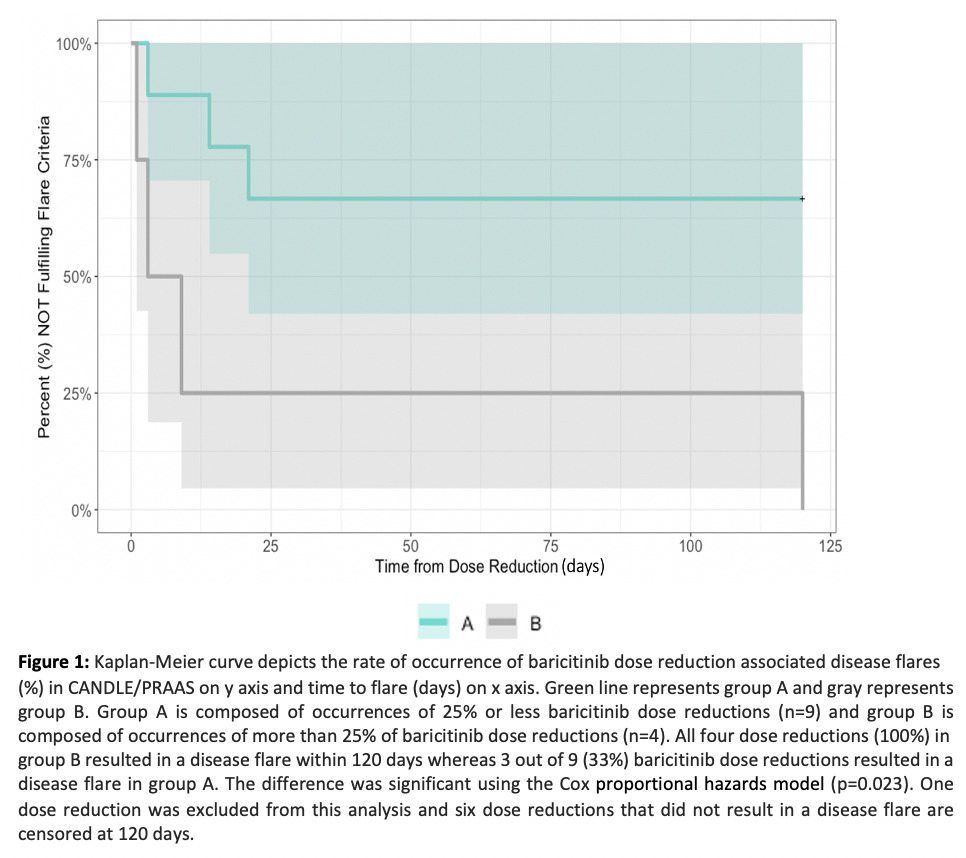
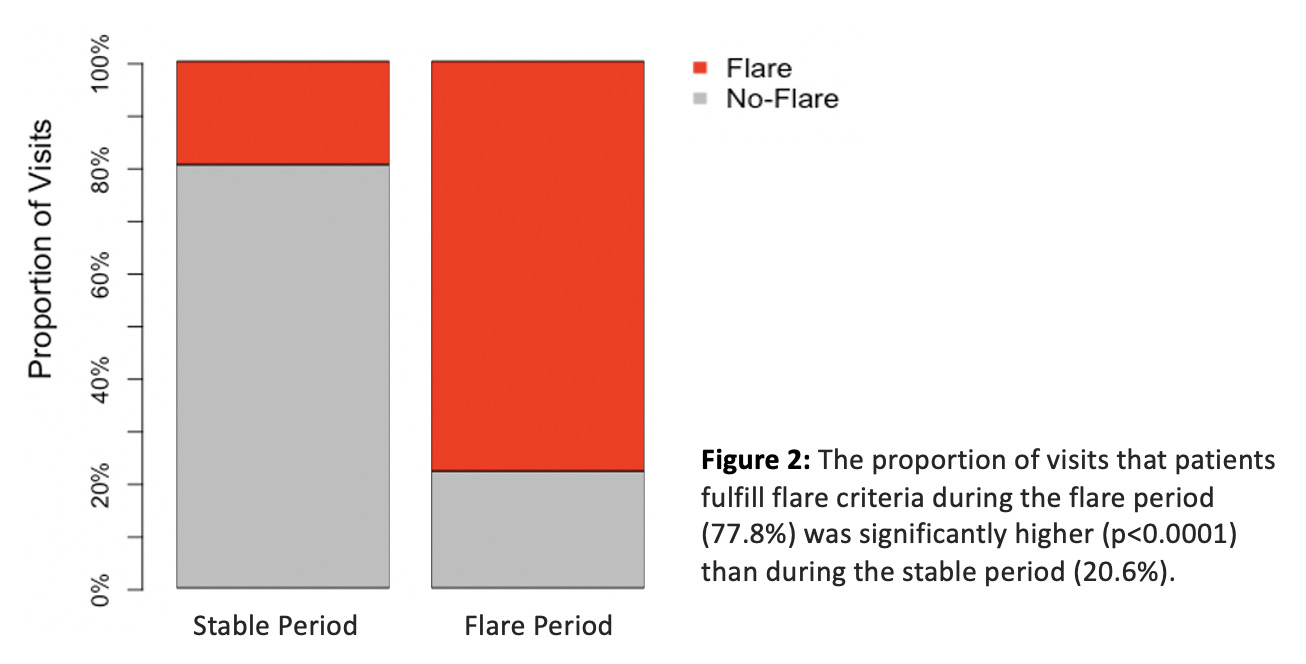
Results: Fourteen baricitinib dose reductions in 9/10 patients occurred and 7/14 (50%) resulted in disease flares in 7/9 patients. A typical flare pattern with acute changes in clinical and/or laboratory biomarkers and cut offs used to define disease flares is outlined in table 1. Time to flare ranged between 1 to 120 days (mean 24 +/- 43 days). A dose reduction of greater than 25% triggered a flare in all patients (figure 1). In patients with baricitinib dose reduction-associated flares, the proportion of visits that patients fulfill flare criteria during the flare period is 77.8% compared to 20.6% during the stable period (p< 0.0001) (figure 2).
Conclusion: We observed significant rebound inflammation and disease flares with >25% dose reductions of baricitinib and propose flare criteria that may be used in quantifying diseases flares and in designing clinical studies in CANDLE/PRAAS. It is important to raise awareness of the development of rebound inflammation with baricitinib dose reductions and the need to closely monitor dose reductions.
Acknowledgements: This baricitinib expanded access program was supported by Eli Lilly. This research was supported by the Intramural Research Program of the NIH, Division of Intramural Research and NIAID.
References:
1. Kim H, et al. Clin Pharmacol Ther. 2018;104(2):364-373.
2. Montealegre Sanchez GA et al. J Clin Invest 2018; 128(7):3041-3052.
To cite this abstract in AMA style:
Cetin Gedik K, Materne G, Ortega-Villa A, Montealegre Sanchez G, Reinhardt A, Brogan P, Berkun Y, Murias S, Robles M, Schalm S, Almeida de Jesus A, Goldbach-Mansky R. Disease Flares in CANDLE/PRAAS with Dose Reductions of Baricitinib [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/disease-flares-in-candle-praas-with-dose-reductions-of-baricitinib/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-flares-in-candle-praas-with-dose-reductions-of-baricitinib/