Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune checkpoint inhibitors (ICI) are effective therapies for patients with cancer, but they often cause immune-related adverse events (irAE). There is limited data on disease flare-ups in patients with pre-existing vasculitis who receive ICI.
Methods: We performed a retrospective review of electronic health records of patients with cancer and a prior history of vasculitis who received ICI at a cancer center. We collected demographics, oncologic history, ICI details (drug, dosage, timing, cycles), characteristics of previous vasculitis, timing and treatment of vasculitis flare, and incidence of other irAEs. Vasculitis was classified according to the 2012 Revised Chapel-Hill Nomenclature (PMID 23045170). Definitions for irAE were based on the 2023 Society for Immunotherapy of Cancer (SITC) consensus (PMID 37001909). Descriptive analysis included means, medians, range and standard deviation (SD) for continuous variables and frequencies and percentages (%) for categorical variables.
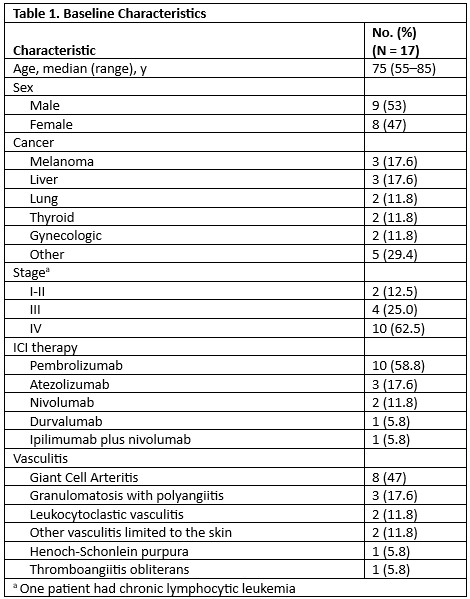
Results: We included 17 patients with prior vasculitis (9 [53%] male, median age 75 years [range 55-85]); 8 had giant cell arteritis (GCA), 3 had granulomatosis with polyangiitis (GPA), 2 had leukocytoclastic vasculitis, 2 had other vasculitis limited to the skin, 1 had Henoch-Schönlein purpura and 1 had thromboangiitis obliterans. Cancer types included melanoma, liver, lung, gynecologic and thyroid cancer. ICI received were: pembrolizumab (10, 59%), atezolizumab (3, 18%), nivolumab (2), durvalumab (1) and ipilimumab plus nivolumab (1). None of the patients had active vasculitis when ICI was initiated; 5 were receiving treatment (3 prednisone, 1 tocilizumab and 1 rituximab).
Only 4 patients (23%; 2 with vasculitis limited to skin, 1 with GCA and 1 with GPA) developed a flare after ICI, in 3 of them biopsy-proven. Only one of the 4 had been receiving treatment at initiation of ICI (prednisone 2.5 mg). One patient with GCA receiving atezolizumab developed proximal muscle stiffness and headaches; temporal artery ultrasound showed signs compatible with vasculitis. Another one with GPA who received pembrolizumab developed glomerulonephritis, sinusitis, and ischemic optic neuropathy. The 2 others with skin vasculitis received pembrolizumab and nivolumab each, and had a flare with purpura and papular rash. Mean disease duration at start of ICI in the patients who flared was 4.3 [SD 1.7] years. Mean time to flare from start of ICI was 18.6 [SD 14.2] weeks. Treatment of the flare included glucocorticoids in 3 patients, combined with sarilumab (GCA flare) and with rituximab (GPA flare), one patient each. All patients were able to continue ICI treatment after flare resolution with vasculitis treatment. Three other patients developed de novo irAEs: Grade 3/4 hepatitis in 2, and warm autoimmune hemolytic anemia in one.
Conclusion: About one in every five patients experienced a vasculitis flare. The outcomes were favorable, as patients responded well to flare treatment, which enabled the continuation of ICI therapy after achieving clinical stability. Prior vasculitis in patients without disease activity should not be considered an absolute contraindication for cancer immunotherapy with ICI.
To cite this abstract in AMA style:
Sevillano J, Zhou Y, Ruiz J, Abdel-Wahab N, Suarez-Almazor M. Disease Flare Following Immune Checkpoint Inhibition in Patients with Cancer and Preexisting Vasculitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/disease-flare-following-immune-checkpoint-inhibition-in-patients-with-cancer-and-preexisting-vasculitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-flare-following-immune-checkpoint-inhibition-in-patients-with-cancer-and-preexisting-vasculitis/