Session Information
The 2020 Pediatric Rheumatology Symposium, originally scheduled for April 29 – May 2, was postponed due to COVID-19; therefore, abstracts were not presented as scheduled.
Date: Saturday, May 2, 2020
Title: Poster Session 3
Session Type: ACR Abstract Session
Session Time: 4:15PM-5:15PM
Background/Purpose: Lupus nephritis associated with childhood-onset systemic lupus erythematosus (cSLE) is a significant risk factor for long-term morbidity and mortality, but little is known regarding current cSLE patients with nephritis in North America. Our objective was to characterize demographics, disease characteristics, and medication utilization patterns from a large multi-center cohort of cSLE patients with nephritis.
Methods: We analyzed data previously collected from March 2017 to June 2019 through the longitudinal, observational cSLE cohort in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. Patients with a cSLE-related condition are eligible for registry enrollment if under 18 at the time of symptom onset, within 24 months of new cSLE diagnosis or new diagnosis of nephritis, and under 21 at the time of enrollment. Data collection occurs every 6 months. Diagnosis of lupus nephritis was defined if at least one renal biopsy was recorded with histopathologic classification by either 1995 World Health Organization (WHO) or 2003 International Society of Nephrology (ISN)/Renal Pathology Society (RPS) criteria. We abstracted the following variables: sex, patient-reported race/ethnicity, insurance status, household income, parent education level, age at diagnosis, reported date of symptom onset, date of diagnosis, date of registry enrollment, Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) at enrollment, physician global assessment (PGA) of disease activity (0-10) at enrollment, date of initial renal biopsy, WHO or ISN/RPS classification of initial renal biopsy, and medications prescribed both prior to and following enrollment. Descriptive statistics were calculated using SAS v9.4.
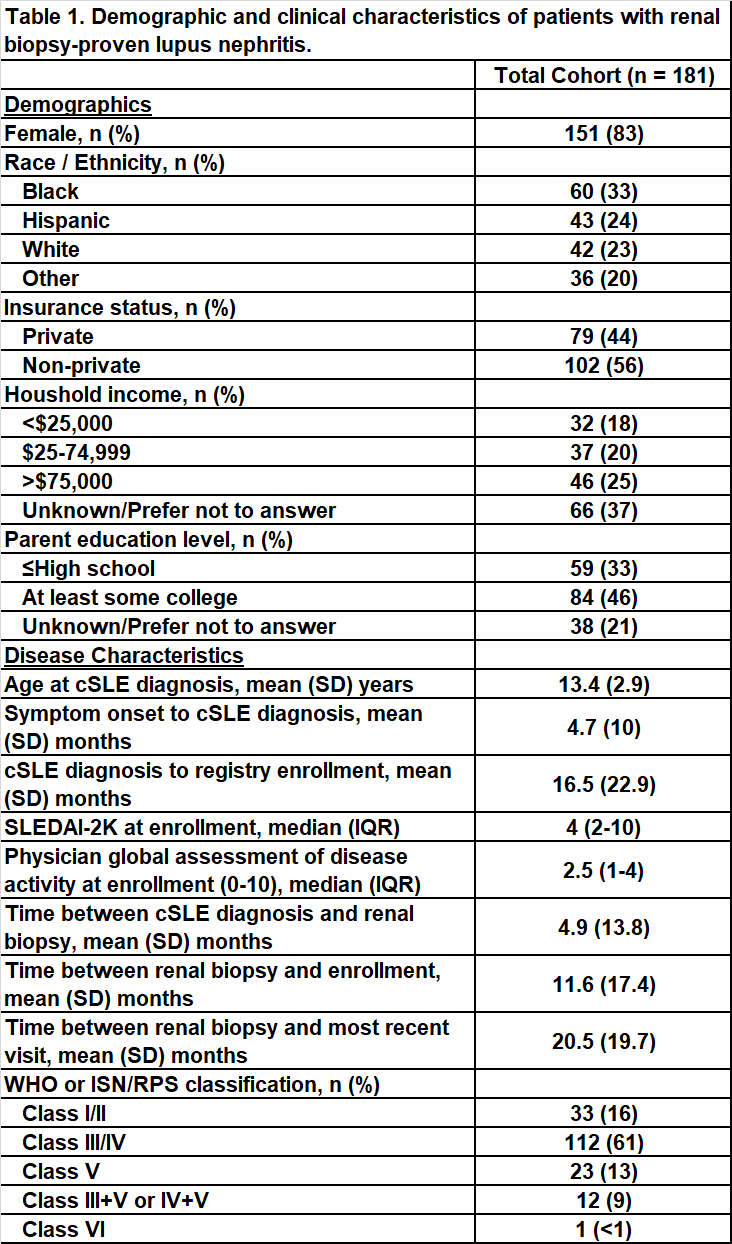
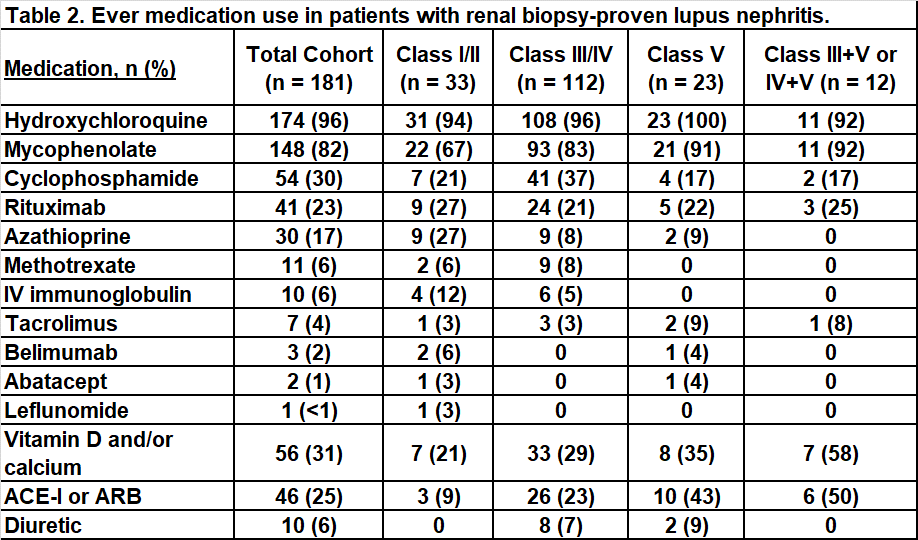
Results: We identified 181 cSLE patients with renal biopsy-proven lupus nephritis from 42 pediatric rheumatology centers. The cohort was 85% female, 33% Black, 24% Hispanic, and 23% White with a mean (SD) age at cSLE diagnosis of 13.4 (2.9) years (Table 1). There was a mean (SD) of 11.6 (17.4) months from time of initial positive renal biopsy to registry enrollment. At enrollment, the median (IQR) SLEDAI-2K was 4 (2-10) and PGA was 2.5 (1-4). On initial biopsy, 16% of patients had class I/II, 61% had class III/IV, 13% had class V, 9% had combined class III/V or IV/V, and 1 patient had class VI. There was high use of hydroxychloroquine and mycophenolate across the cohort, while cyclophosphamide and azathioprine use varied by biopsy classification (Table 2). Across 12 centers with at least 5 patients with nephritis enrolled, wide variation in use of mycophenolate (58-100%), cyclophosphamide (0-86%), and rituximab (0-100%) was observed (Figure 1).
Conclusion: Use of the CARRA Registry provides a large multi-center sample of cSLE patients with lupus nephritis from which to evaluate disease characteristics and medication utilization patterns. This initial analysis demonstrates a diverse cohort of patients with differential medication use patterns across nephritis classification groups and care centers. Continued study of optimal care for cSLE with nephritis is needed to reduce disparities and improve long-term outcomes.
To cite this abstract in AMA style:
Smitherman E, Chahine R, Beukelman T, Lewandowski L, Rahman A, Wenderfer S, Hersh A, Curtis J. Disease Characteristics and Medication Utilization in Lupus Nephritis Associated with Childhood-Onset Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 4). https://acrabstracts.org/abstract/disease-characteristics-and-medication-utilization-in-lupus-nephritis-associated-with-childhood-onset-systemic-lupus-erythematosus/. Accessed .« Back to 2020 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-characteristics-and-medication-utilization-in-lupus-nephritis-associated-with-childhood-onset-systemic-lupus-erythematosus/