Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The goal of treatment for patients with juvenile idiopathic arthritis (JIA) is inactive or minimal disease activity and escalating medication for active disease is recommended. The goal of this analysis is to assess patterns of disease activity at 2 sequential registry visits and associated new medication start.
Methods: Patients with JIA enrolled in the Childhood Arthritis and Rheumatology Research Alliance Registry, a North American multicenter registry, with complete clinical Juvenile Arthritis Disease Activity Scores (cJADAS) at the 6 month and 12-month registry visits were included. Disease activity at each visit was classified according to cJADAS categories (inactive, minimal, moderate, or high) for either oligo- or polyarticular disease course, regardless of JIA categorization. Patient characteristics were determined at the 12-month Registry visit. The primary outcome was new medication (methotrexate or biologic) start at the 12-month visit determined by a clinical site attestation. Stratifying by the respective pairings of 6- and 12-month cJADAS categories, we examined the association between paired cJADAS values, disease activity, and medication changes.
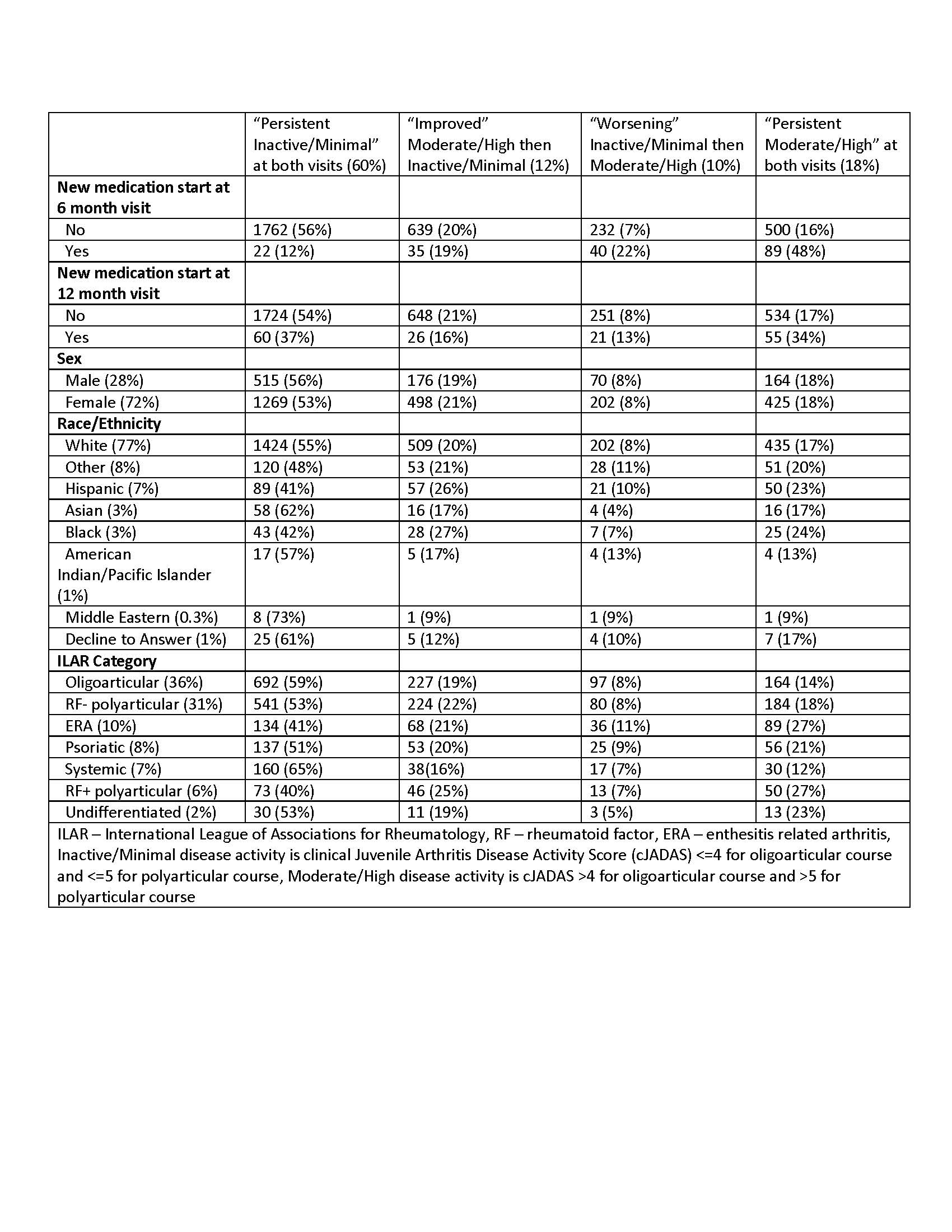
Results: Our sample included 3325 patients with JIA: 72% were female, 77% were white, 91% were from the US, and two-thirds had oligoarticular (36%) or rheumatoid factor (RF)- polyarticular JIA (31%). The patterns of disease activity across paired visits were: 54% persistent inactive/minimal, 18% persistent moderate/high, 20% “improved” (moderate/high then inactive/minimal), and 8% “worsening” (inactive/minimal then moderate/high)(Table 1). Only 6% of all patients had a new medication start attested after the 6-month Registry visit and 5% at the 12-month visit. A higher percentage of patients with moderate/high disease activity at the 12-month visit started a new medication (9%) compared to those with inactive/minimal disease activity at the 12-month visit (3%) (X2 p< 0.0001). Of those starting a new medication at the 12-month visit, 37% had persistent inactive/minimal disease activity. Notably, 91% of patients with persistent moderate/high disease activity had no medication change at the 12-month visit.
Conclusion: In a large multicenter registry of patients with JIA, starting a new medication was more common for those with moderate/high disease activity at the 12-month visit, but was still uncommon overall. Interestingly, starting a new medication occurred for some patients with persistent inactive/minimal disease and did not occur for most patients with persistent moderate/high disease activity. Our findings suggest that starting a new medication is not driven by longitudinal disease activity scores. Further study is needed to identify reasons for treatment non-escalation among patients with persistent moderate or high disease activity.
To cite this abstract in AMA style:
Mannion M, Aswani M, Hearld K, Smitherman E, Huie L, Curtis J. Disease Activity and New Medication Start at Two Consecutive Registry Visits for Patients with Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/disease-activity-and-new-medication-start-at-two-consecutive-registry-visits-for-patients-with-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-activity-and-new-medication-start-at-two-consecutive-registry-visits-for-patients-with-juvenile-idiopathic-arthritis/