Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Haematological manifestations of SLE are common with anaemia reported in approximately 50% of cases, yet the role of altered iron metabolism is poorly understood. Key regulators of iron homeostasis include; 1. Hepcidin, which prevents iron release from stores (under the influence of IL6 and IL1β); 2, Ferritin, an iron carrier protein also implicated as a functional potent pro-inflammatory mediator; 3. Erythropoietin (EPO); 4. Lipocalin-2 (LCN2), released on innate immune activation that induces iron sequestration; 5. Transferrin, binds circulating iron and regulates transport to stores in the liver, spleen and bone marrow; 6. Soluble transferrin receptor (sTfR), a cleaved extracellular receptor; 7. Haptoglobin (Hp), binds free haemoglobin (Hb); 8. Natural resistance-associated macrophage protein 2 (NRAMP2), a metal ion transporter that facilitates transfer of iron into enterocytes.
In this study, we aimed to evaluate the changes in these regulators in patients with SLE and assess for the influence of disease activity for the first time.
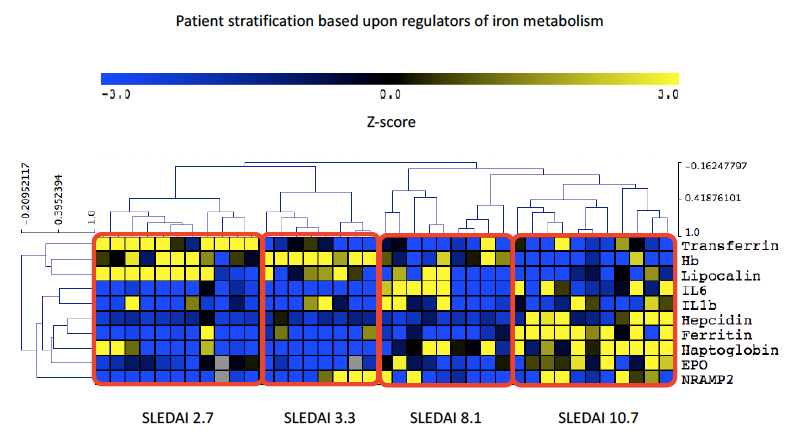
Methods: Serum samples were collected from patients with SLE (n=39) attending University College London Hospital, UK, in addition to 17 healthy controls (HC). Patients with haemolytic anaemia were excluded. Clinical measures including Hb, dsDNA, complement C3 and SLEDAI-2K (where >4 was considered active disease; ≤4 classified inactive / low disease activity) were recorded. Levels of IL6, IL1β, Hepcidin, Ferritin, LCN2, Transferrin, sTfR, EPO, Hp and NRAMP2 were measured by ELISA. Differences between groups were calculated by One-way ANOVA / Kruskal-Wallis and correlations by Spearman’s / Pearson’s. Following normalisation of significant variables, Z-scores were used in hierarchical correlate clustering performed using MeV software to present as a heatmap.
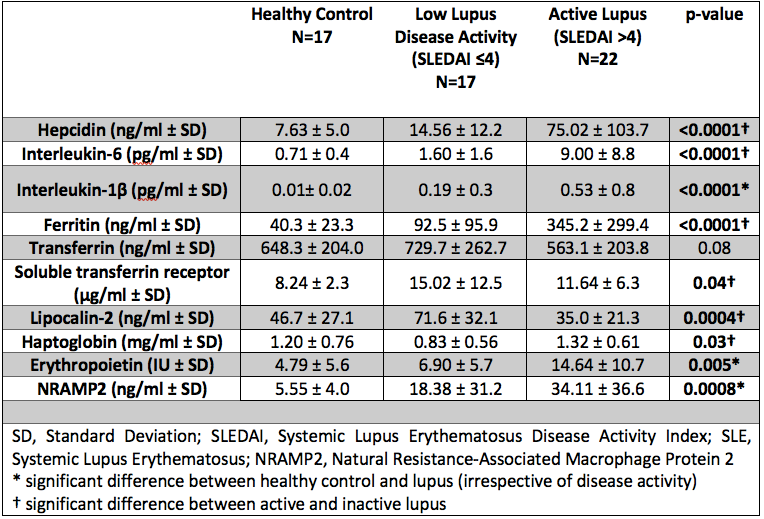
Results: Of the 39 patients recruited 90% were female (35/39) with a mean age of 27.8 (± 11.7) years. Results comparing HC, inactive / low disease activity SLE and active SLE are summarised in Table 1. Interestingly LCN2 levels were lower in active SLE, which is surprising given that elevated levels are commonly observed in systemic inflammation. SLEDAI-2K showed a strong positive correlation with hepcidin, ferritin, IL6 and Hp, whilst negatively correlating with both LCN2 and Transferrin. In SLE, ferritin and LCN2 correlated significantly regardless of disease activity (p< 0.001, r=0.40). Figure 1 summarises all correlations in SLE. Figure 2 demonstrates the results of hierarchical clustering. The cluster containing patients with a combination of reduced LCN2 and Hb with elevated Hp, Ferritin, Hepcidin and EPO showed the highest mean SLEDAI-2K (10.7) whilst higher Hb, LCN2 and Transferrin was observed in the group with the lowest mean SLEDAI-2K (2.7).
Conclusion: We have detailed the dysregulation of key mediators in iron homeostasis in SLE for the first time. Key findings include a paradoxical reduction in LCN2, which inversely correlates with ferritin. Further, reduced Transferrin with elevated EPO and Hepcidin in active disease suggests a state of functional iron deficiency with iron being held in stores and therefore not available for use in erythropoiesis thus representing a novel mechanism for anaemia.
To cite this abstract in AMA style:
Wincup C, McDonnell T, Robinson G, Farinha F, Radziszewska A, Rahman A. Disease Activity and Dysregulated Iron Metabolism: A Potentially Overlooked Mechanism for Anaemia in Patients with Systemic Lupus Erythematosus? [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/disease-activity-and-dysregulated-iron-metabolism-a-potentially-overlooked-mechanism-for-anaemia-in-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-activity-and-dysregulated-iron-metabolism-a-potentially-overlooked-mechanism-for-anaemia-in-patients-with-systemic-lupus-erythematosus/