Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Early response to anti-TNF therapy has been shown to be a strong predictor of good long-term outcomes in ankylosing spondylitis (AS).1 However, early identification of patients (pts) unlikely to achieve good long-term disease control by anti-TNF therapy has been less well characterized, although identifying such pts may help avoid unnecessary exposure, increase cost-effectiveness and improve the chance of achieving long-term treatment goals. Here we aim to assess the association between disease activity (DA) during the first 12 weeks (wks) of treatment, and attainment/lack of attainment of treatment targets at Wk48 in axial spondyloarthritis (axSpA) pts, including AS and non-radiographic (nr-) axSpA pts, receiving certolizumab pegol (CZP).
Methods: The relationship between DA during the first 12 wks of treatment and achievement of the Wk48 treatment targets: ASDAS Inactive Disease (ID) or BASDAI <2 with or without CRP levels equal to or below the upper limit of normal (ULN=7.9mg/L), was assessed post hoc using CZP data from the RAPID-axSpA trial (NCT01087762).2 DA state was defined for ASDAS as: ID, moderate (MD), high (HD) or very high DA (vHD), and for BASDAI as Low (<2), Moderate (≥2 to <4), High (≥4 to ≤6) or Very High (>6). BASDAI thresholds have not been validated. Analyses are based on all pts randomized to CZP (200mg Q2W or 400mg Q4W) in the overall axSpA population and also the AS and nr-axSpA subpopulations. Predictability analyses for a given wk are based on all pts continuing treatment at that wk. For these pts, LOCF was applied for withdrawals before, or missing evaluation at, Wk48.
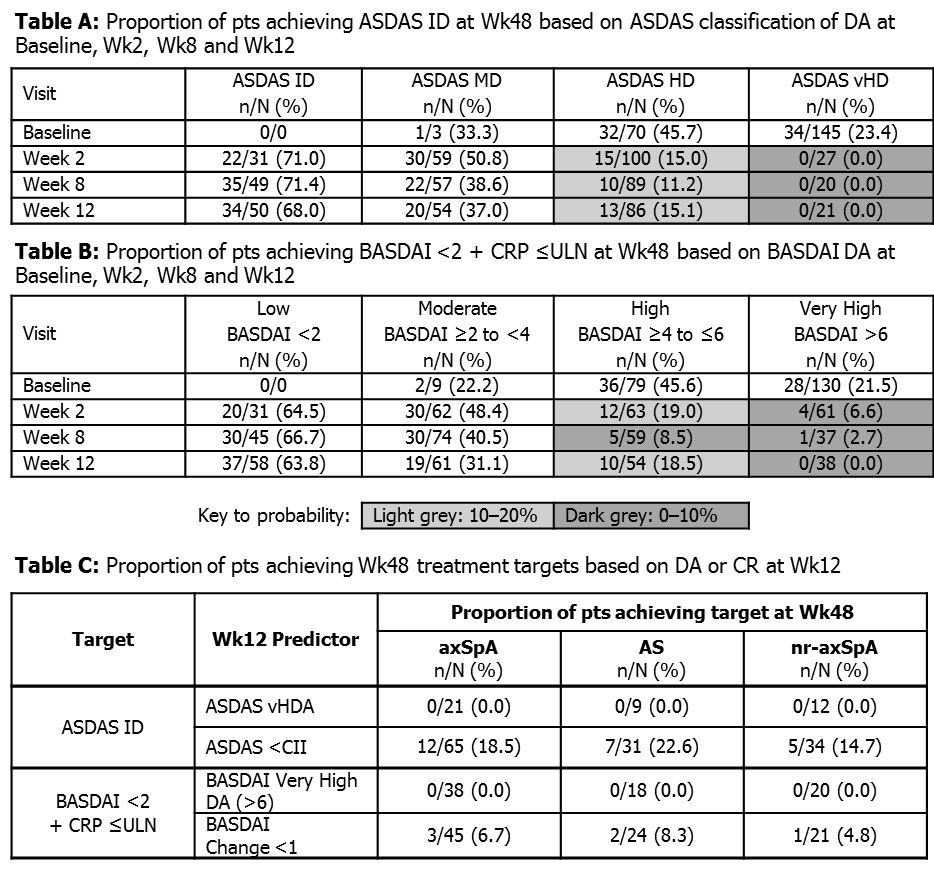
Results: ASDAS DA state at Wk2 was associated with likelihood of achieving ID at Wk48, with 71% (22/31) of pts with ID at Wk2 achieving ID at Wk48, compared with 0% (0/27) of pts with vHD at Wk2 achieving ID at Wk48. A similar trend was observed at Wk12, although fewer pts had HD and vHD, and more pts had ID at this time point (Table A). BASDAI Very High DA also successfully predicted the lack of attainment of the treatment target BASDAI <2 + CRP ≤ULN (Tables B and C), and results were not altered if only BASDAI <2 (and not CRP level) was the target. Lack of clinical response (CR) to CZP was also an effective negative predictor of Week 48 DA (Table C), with 3/45 (6.7%) pts with Wk12 BASDAI improvement <1 achieving Wk48 DA of BASDAI <2, and 12/65 (18.5%) pts with Wk12 ASDAS improvement less than clinically important improvement (
References:
1. Sieper J. Ann Rheum Dis 2012;71:700-706
2. Landewé R. Ann Rheum Dis 2014;73:39-47
Disclosure:
D. M. van der Heijde,
AbbVie, Amgen, AstraZeneca, Augurex, BMS, Celgene, Centocor, Chugai, Covagen, Daiichi, Eli-Lilly, Galapagos, GSK, Janssen Biologics, Merck, Novartis, Novo-Nordisk, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Vertex,
5,
Imaging Rheumatology bv,
9;
A. A. Deodhar,
Abbott, Amgen, Janssen, Novartis, Pfizer and UCB Pharma,
2,
Abbott, Amgen, Janssen, Novartis, Pfizer and UCB Pharma,
5;
O. Davies,
UCB Pharma,
3,
UCB Pharma,
1;
T. Nurminen,
UCB Pharma,
3;
M. Rudwaleit,
Abbott, BMS, MSD, Pfizer, Roche, UCB Pharma,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-activity-and-clinical-response-early-in-the-course-of-treatment-predict-long-term-outcomes-in-axial-spondyloarthritis-patients-treated-with-certolizumab-pegol/