Session Information
Date: Sunday, November 8, 2015
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Discordance between patient (pt) and physician (phy) assessment of disease activity (DA) in spondyloarthritis (SpA) is recognized, with phys tending to score DA less severely than pts.1Perceptual differences need to be understood to improve pt-phy dialogue, set shared treatment goals and ultimately improve outcomes. The objectives were to: 1. Investigate the proportion of axial SpA (axSpA) pts with acceptable symptomatic states as scored by the pt or their phy before and after initiation of anti-TNF treatment and 2. Explore discrepancies between pt and phy assessments.
Methods:
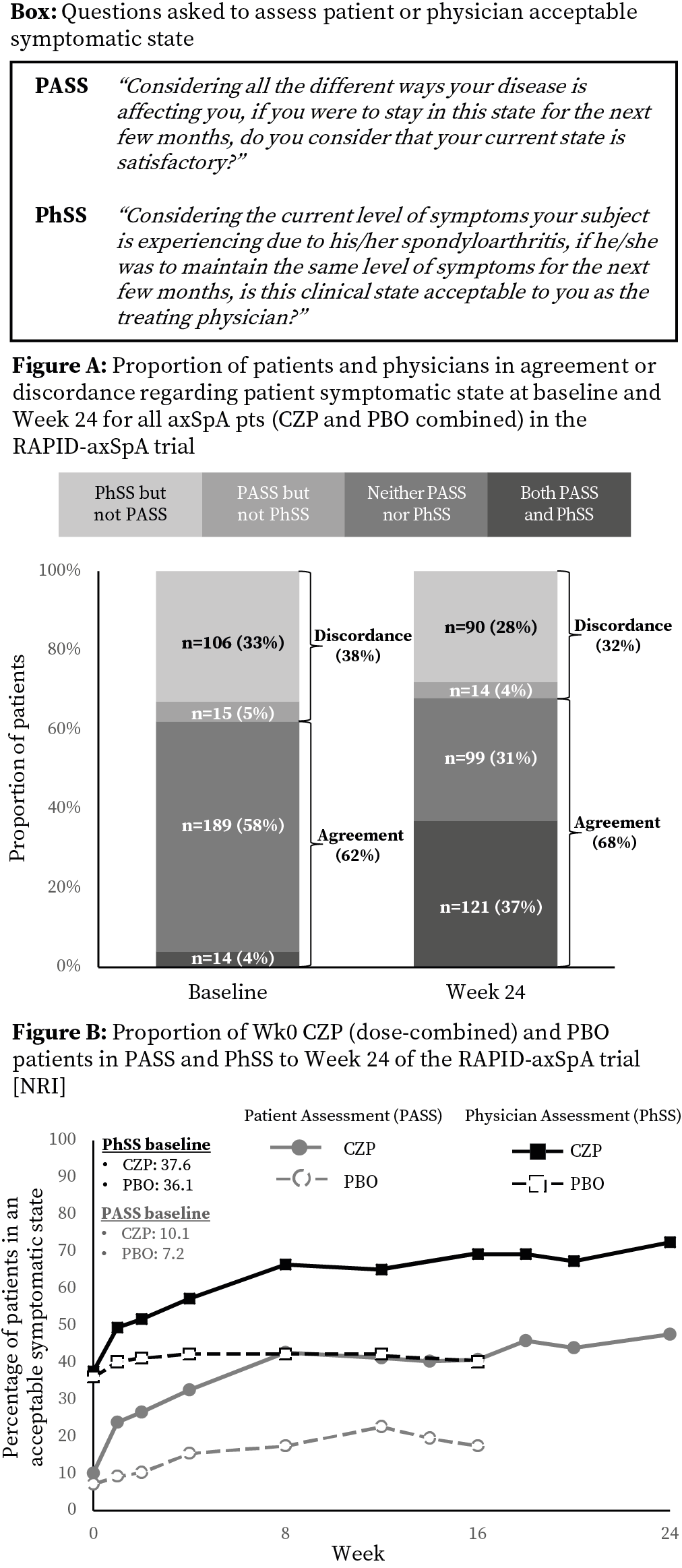
RAPID-axSpA (NCT01087762) was double-blind and placebo (PBO) controlled to Week (Wk) 24.2Pts fulfilled ASAS criteria and had active axSpA. At baseline (BL) 325 pts were randomized 1:1:1 to PBO, or certolizumab pegol (CZP) 200 mg Q2W / 400 mg Q4W. In addition to conventional axSpA outcomes (eg ASDAS / BASDAI) information on pt acceptable symptomatic state was collected from the pt (Pt Acceptable Symptomatic State [PASS]) and their phy (Phy Acceptable Symptomatic State [PhSS]) (see Box for questions).
Analyses also investigated underlying DA thresholds considered ‘acceptable’ by pts or phys. Based on pt’s global assessment of DA (PtGADA) and PASS, interval-censored observations estimated each pt’s ‘acceptability threshold’. Assuming normal distribution, the mean was estimated using LIFEREG in SAS (v 9.3). Similar procedure was used for phys.
Results:
Of 324 pts assessed, 218 received CZP from Wk0 and 106 PBO. At BL 29 (9%) pts were in PASS and 120 (37%) in PhSS, despite high DA in pts with PhSS (mean ASDAS 3.8; BASDAI 6.4). Similar proportions of pts and phys agreed regarding pt symptomatic state at BL (62%) and Wk24 (68%; Fig A); this was similar for CZP and PBO (BL CZP 62%, PBO 63%; Wk24 CZP 67%, PBO 70%).
Improvements following CZP treatment (dose combined) were seen from Wk1 (Fig B). Differences in proportions of pts in PASS vs PhSS at BL were maintained to Wk24, with 104 (48%) CZP treated pts in PASS and 158 (73%) in PhSS (Fig B). At Wk24 mean underlying DA level considered ‘acceptable’ was similar for pts and phys and was not dependent on treatment (PtGADA CZP 3.9 vs PBO 3.7; PhGADA CZP 3.9 vs PBO 4.0).
Preliminary analyses investigating BL characteristics indicate that pts with Wk24 PASS had lower BL DA scores than pts without; no differences were seen for pts in / not-in PhSS at BL or Wk24.
Conclusion:
CZP treatment was associated with improvements in PASS and PhSS to Wk24. Despite high DA at BL over a third of phy scored pts as having acceptable symptomatic states, which represented a notable discrepancy compared to pt scoring. This discrepancy was maintained to Wk24 and was independent of treatment. These findings confirm previous reports of discordance in phy and pt assessment of DA and highlight a need for closer partnership between phys and pts to set shared treatment goals.
References:
- Desthieux C. Ann Rheum Dis 2015;74(S2):498
- Landewé R. Ann Rheum Dis 2014;73:39–47
To cite this abstract in AMA style:
Dougados M, Davies O, Nurminen T, Coteur G, Gossec L. Discrepancy Between Patients and Physicians Acceptable Symptomatic States in Axial Spondyloarthritis: Findings from the RAPID-AxSpA Study [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/discrepancy-between-patients-and-physicians-acceptable-symptomatic-states-in-axial-spondyloarthritis-findings-from-the-rapid-axspa-study/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discrepancy-between-patients-and-physicians-acceptable-symptomatic-states-in-axial-spondyloarthritis-findings-from-the-rapid-axspa-study/