Session Information
Date: Monday, November 18, 2024
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Glucocorticoids (GC) have a rapid effect on symptoms, but chronic exposure is associated to important adverse events. Although both EULAR and ACR guidelines (1,2), recommend to use the lowest effective dose for less than three months as bridging therapy, prednisone is used often for periods longer than six months in clinical practice. The greater availability of advanced therapies should help physicians to discontinue GC when low disease activity is achieved. We aim to compare the GC-sparing effect of biologic and targeted synthetic DMARDs (b/tsDMARD) among patients with rheumatoid arthritis (RA) in a real-life multicenter cohort and to identify predictors associated to GC discontinuation.
Methods: A total of 3,384 RA patients from a multicenter cohort initiating a first line treatment with a b/tsDMARD -TNFi, anti-IL6, anti-CD20, JAKi, and CTLA4-Ig- were identified. Concomitant GC use at treatment initiation was 56.4%. As TNFi was the larger group, a random sample was taken for comparison purposes. Patients with at least one-year follow-up were included in the analysis. The main outcome was GC discontinuation after one-year, assessed with a multivariate logistic regression. Baseline characteristics were analysed according to GC status. Kaplan-Meier estimates and Cox regression, adjusted for patient, disease and treatment characteristics, were used to evaluate GC discontinuation in patients with GC at baseline.
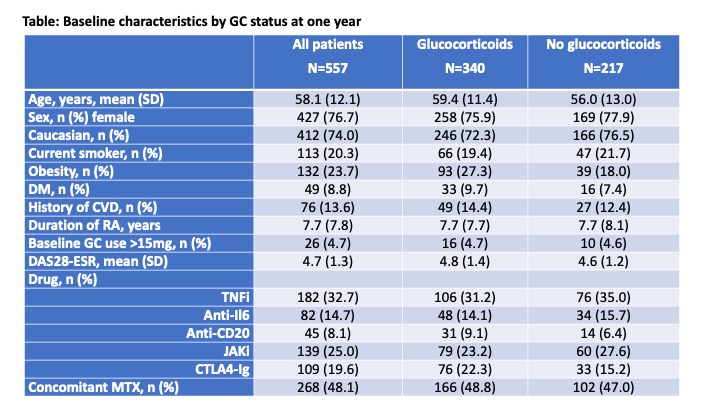
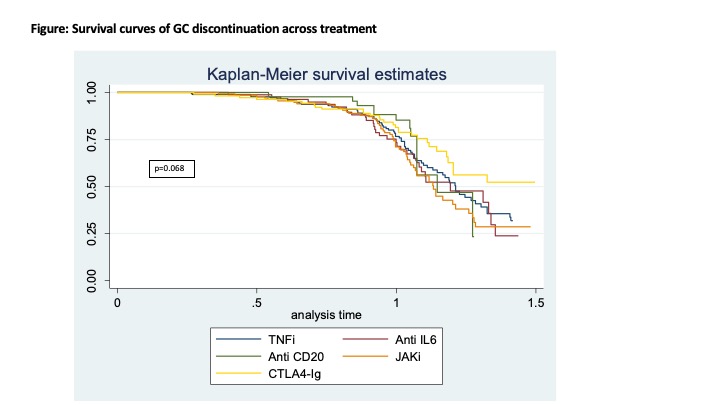
Results: A total of 557 RA patients were included, 182 treated with TNFi, 82 with anti-IL6, 45 with anti-CD20, 139 with JAKi, and 109 with CTLA4-Ig. At one-year, GC use decreased similarly in all treatment groups compared to TNFi [odds ratio for discontinuation 0.95 (0.53 – 1.70) for anti-IL6, 0.81 (0.39 – 1.69) for anti-CD20, 1.07 (0.65 – 1.75) for JAKi, and 0.74 (0.41 – 1.31) for CTLA4-Ig]. There were no differences at baseline according to GC status after one year (Table). The proportion on GC was reduced in a gradual manner over time (Figure 1), and the proportion of patients with GC discontinuation after 1-year follow-up was: 41.7% for TNFi, 41.5% for anti-IL6, 31.1% for anti-CD20, 43.2% for JAKi, and 30.3% for CTLA4-Ig. Compared to TNFi, adjusted hazard ratios were 1.09 (0.70-1.70) for anti-IL6, 1.07 (0.59-1.95) for anti-CD20, 1.31 (0.91-1.91) for JAKi, and 0.80 (0.51-1.28) for CTLA4-Ig.
Conclusion: Our data showed that GC were frequently prescribed when initiating b/tsDMARDs, and still used after 1-year follow-up in many patients. Only between 30% with CTLA4-Ig4 and 43% with JAKi patients discontinued GC after one-year follow-up in our cohort. We did not identify any predictor of discontinuation at baseline.
To cite this abstract in AMA style:
Castrejon I, Otero-Varela L, Álvaro-Gracia J, Calvo Gutierrez J, Campos-Fernández C, García Dorta A, Perez A, RUIZ MONTESINO D, Sánchez-Alonso F. Discontinuation Pattern of Glucocorticoids in Patients with Rheumatoid Arthritis Initiating Biologics or Targeted Synthetic DMARDs in Routine Care [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/discontinuation-pattern-of-glucocorticoids-in-patients-with-rheumatoid-arthritis-initiating-biologics-or-targeted-synthetic-dmards-in-routine-care/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-pattern-of-glucocorticoids-in-patients-with-rheumatoid-arthritis-initiating-biologics-or-targeted-synthetic-dmards-in-routine-care/