Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose:
In the treatment of rheumatoid arthritis (RA), glucocorticoid that provide anti-inflammatory effects is an important drug. We recommend discontinuing of glucocorticoid as soon as possible, but many patients have taken oral glucocorticoid for the long term in daily clinical practice. On the other hand, there are several reports on discontinuation of glucocorticoid due to the initiation of biological disease-modifying antirheumatic drugs (bDMARD). The aim of this study is to examine association of methotrexate (MTX) dose with discontinuation of glucocorticoid after 52 weeks since initiation of 1st bDMARDs.
Methods: This study was based on data from a Japanese multicenter registry, Tsurumai Biologics Communication Registry (TBCR), and enrolled 3119 patients who used bDMARDs from 2008 Oct to 2015 Oct. We examined the status of oral glucocorticoid use after 52 weeks since initiation of bDMARDs as 1st bDMARDs. In predictive analyses, discontinuation of glucocorticoid after 52 weeks since initiation of bDMARDs was used as outcome variable. Factor associated with baseline characteristics at the time of initiation of bDMARDs was assessed with univariate and stepwise forward multivariate logistic regression. Propensity score matching (PS) matching was used to align the patients background with selection bias in observational studies. The adjustment variables were age, disease duration, sex, disease activity, seropositivity, and glucocorticoid dose.
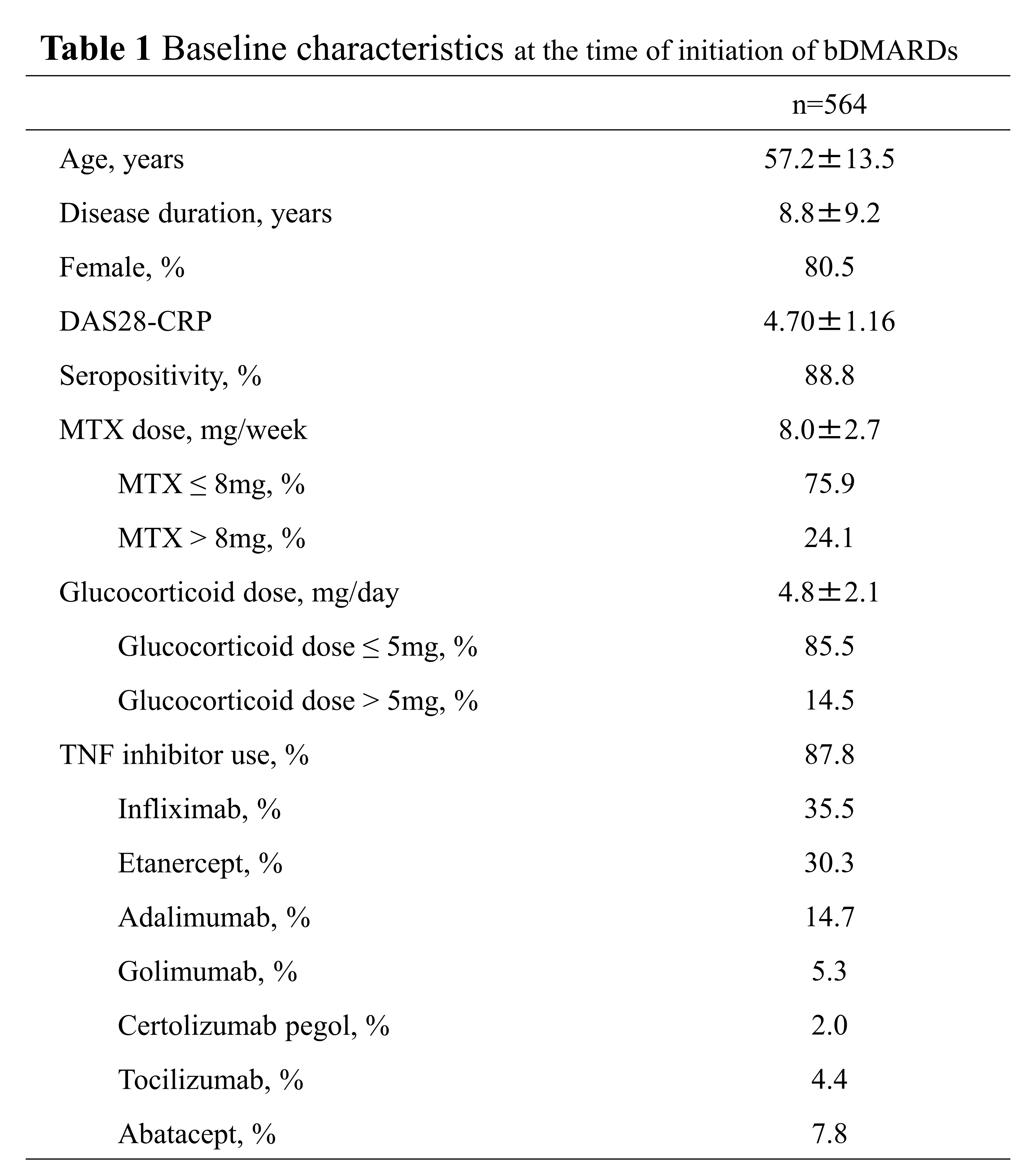
Results: 564 patients who used glucocorticoid and MTX when bDMARDs was initiated as 1st bDMARDs were included (Table 1). 164 cases (29.1%) were discontinued glucocorticoid up to 52 weeks after initiation of bDMARDs. In the multivariate analysis, age (odds ratio (OR) 0.98), MTX dose (OR1.11) and glucocorticoid dose (OR0.87) were independently predictive factors of discontinuation of glucocorticoid at the time of initiation of bDMARDs (Table 2). When we adjusted factors of baseline characteristic by using propensity score matching of the patients group taking MTX≤8mg and MTX >8mg, 105 pairs were extracted (Table 3). In the patients group taking MTX >8 mg, discontinuous rate of glucocorticoid (41.0%) was significantly higher. Regarding the clinical course, DAS28-CRP at baseline was 4.53 in the group of patients taking MTX >8 mg, and 4.51 in the group of patients taking MTX≤8 mg, and there was no obvious significant difference. At 52 weeks, 2.87 in the group of patients taking MTX >8 mg, and 2.59 in the group of patients taking MTX≤8 mg, and there was also no obvious significant difference. Further, there was no obvious significant difference in the rate of change in disease activity.
Conclusion: This cohort study investigated the association with discontinuation of oral glucocorticoid and MTX dose in the patients treated with bDMARDs. In the clinical practice, MTX dose at the time of initiation of bDMARDs was predictive factor of discontinuation of glucocorticoid. A higher dose of MTX associated with discontinuation of glucocorticoid in the patients treated with bDMARDs. Glucocorticoid use suggested that it could be decreased or discontinued in the patients treated with bDMARDs.
bDMARDs, biological disease-modifying antirheumatic drugs; DAS28, disease activity score in 28 joints; CRP, serum c-reactive protein; MTX, methotrexate; TNF, tumor necrosis factor.
95% CI, 95% confidence interval; bDMARDs, biological disease-modifying antirheumatic drugs; DAS28, disease activity score in 28 joints; CRP, serum c-reactive protein; MTX, methotrexate; TNF, tumor necrosis factor. *P < 0.05
bDMARDs, biological disease-modifying antirheumatic drugs; MTX, methotrexate; DAS28, disease activity score in 28 joints; CRP, serum c-reactive protein. *Student’s t test, **chi-squared test.
To cite this abstract in AMA style:
Suzuki M, Kojima T, Takahashi N, Asai S, Ishiguro N. Discontinuation of Oral Glucocorticoid After Initiation of Biological DMARDs Due to a Higher Dose of Methotrexate; A Retrospective Observational Study Based on Data from a Japanese Multicenter Registry Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/discontinuation-of-oral-glucocorticoid-after-initiation-of-biological-dmards-due-to-a-higher-dose-of-methotrexate-a-retrospective-observational-study-based-on-data-from-a-japanese-multicenter-registr/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-of-oral-glucocorticoid-after-initiation-of-biological-dmards-due-to-a-higher-dose-of-methotrexate-a-retrospective-observational-study-based-on-data-from-a-japanese-multicenter-registr/