Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The treatment of Rheumatoid Arthritis (RA) has undergone a big change in the last two decades with the Disease Modifying Drugs (DMARDs). These drugs are widely used in clinical practice, but we have little information about their discontinuation in this conditions especially due to inefficacy. Purpose: to describe the discontinuations of DMARDs due to inefficacy, their causes in patients with incident RA, and to evaluate possible associated factors to inefficacy.
Methods: We conducted an observational longitudinal study. Patients: all recent onset RA diagnosed between January 1st 2007 and December 31st 2015 followed in outpatient clinic at Hospital Clinico San Carlos until January 1st 2017, which used any DMARD (synthetic and biologic) during at least 3 months. Primary outcome: discontinuation of the DMARD due to inefficacy. Covariables: sociodemographic, clinical and treatments. Incidence rates of discontinuation (IR) per 100 patient-years were estimated using survival techniques with their respective 95% confidence interval [CI]. Factors associated with DMARDs discontinuation due to inefficacy was performed using Cox regression models, and results were expressed in Hazard ratio (HR) and [CI].
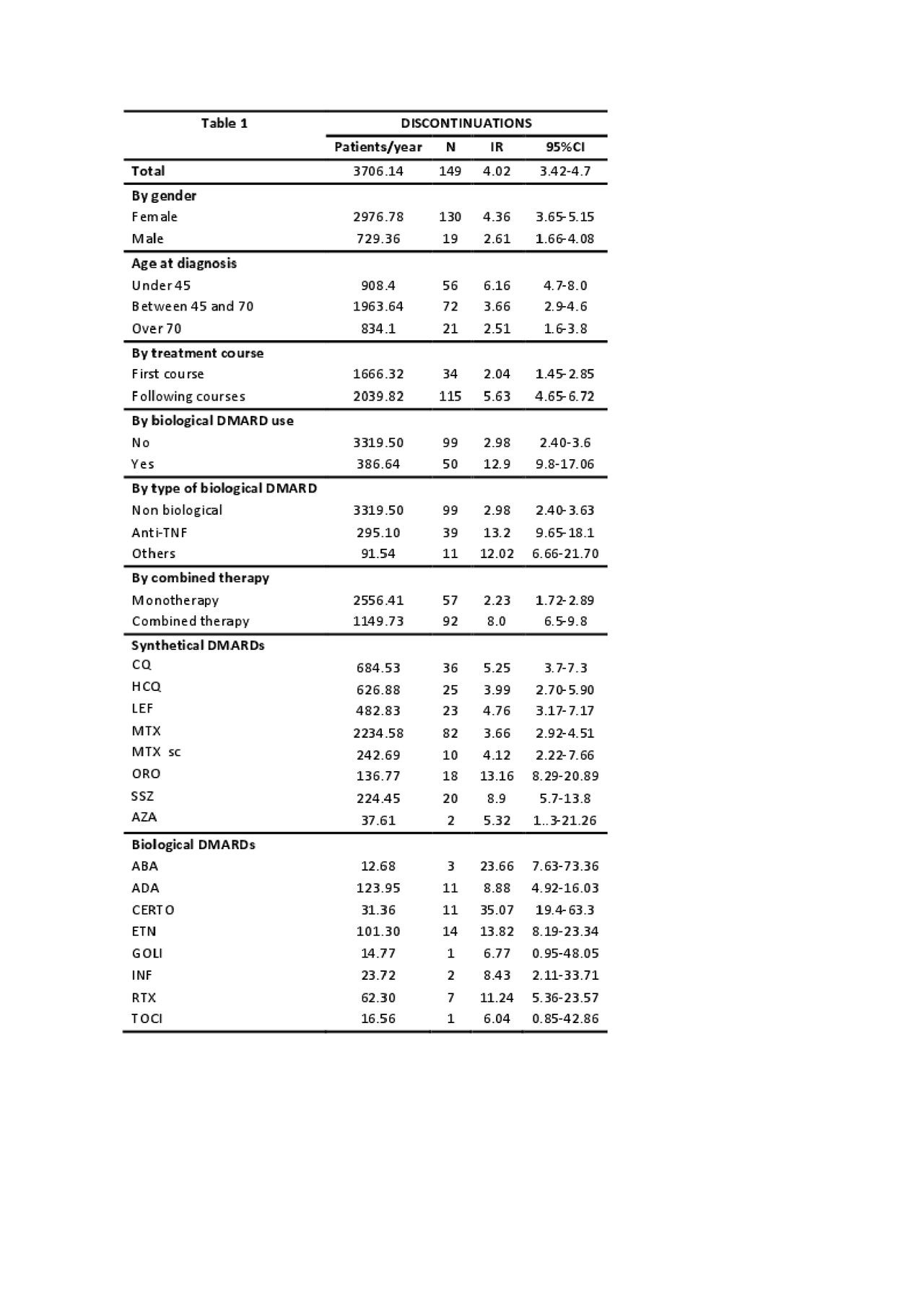
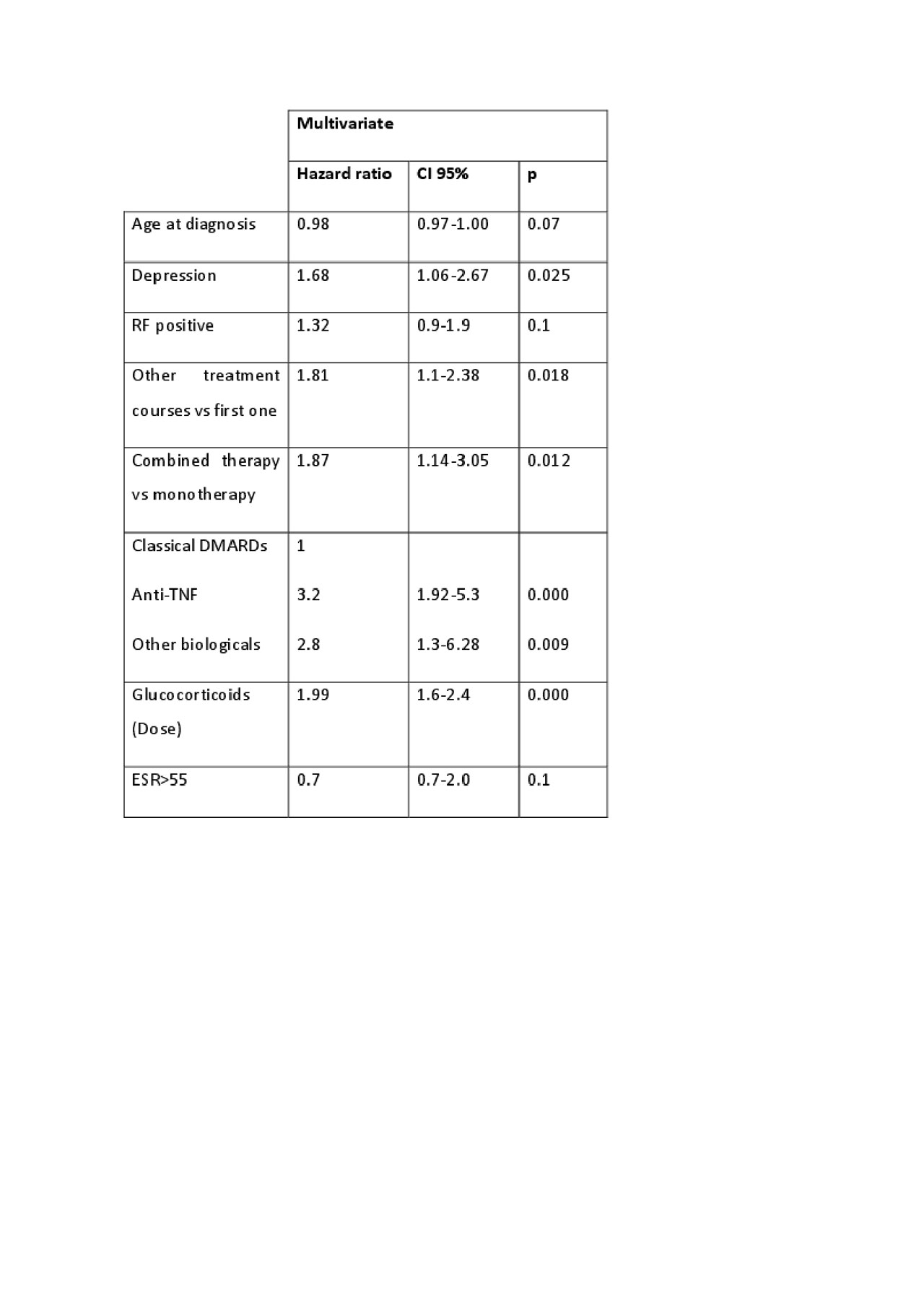
Results: We included 2388 courses of DMARD treatment in 814 patients (3706.14 patient-years). 77.52% were women with a mean age at diagnosis of 57.53±15.50 years. There were 1094 DMARDs discontinuations of the 2388 courses (45.81%) in 438 patients, of these 149 in 110 patients (13.5%) were due to inefficacy (IR: 4.0[3.4-4.7]). Most patients discontinuing due to inefficacy were women (87.50%) with a mean age at diagnosis of 52.40±13.50 years. 33.5% of the discontinuations due to inefficacy were biological DMARDs. 61.7% were in combined therapy and most of them (77.18%) discontinued during successive courses of therapy. IRs are shown in table 1. The multivariate analysis (table 2) was adjusted by age, gender and disease activity (ESR). Interestingly, younger age and depression also increased the risk of inefficacy. Combined therapy, second courses, dose of glucocorticoids and biologic vs synthetic DMARDs (HR: 3.12 [1.8-5.2]) increased the risk. We repeated the multiple regression model with all the sociodemographic and clinical variables shown in table 2 but changing the reference category of DMARD and found that Certolizumab (HR: 5.0[2.5-9.8]) had the highest risk of discontinuation due to inefficacy compared to the other drugs followed by Abatacept (HR: 4.9[1.9-12.7]), Etanercept (HR: 2.3[1.3-4.2]) and Gold (HR: 1.9 [1.1-3.4]). MTX use (HR: 0.6 [0.42-0.87]) was a protective factor against discontinuation due to inefficacy.
Conclusion: Conclusions. Discontinuations of DMARDs in clinical practice due to inefficacy are frequent (13.5%) with an IR of 4%. We have found differences in discontinuation rates among DMARD with Certolizumab, Abatacept, Etanercept, and Gold being the drugs with the highest risk. MTX is a protective factor for discontinuation due to inefficacy. Caution should be taken in regard to discontinuation due to inefficacy in patients receiving glucocorticoids, biological DMARDs, combined therapy, in subsequent treatment courses, and those with depression.
To cite this abstract in AMA style:
Rosales Rosado Z, Lois p, Mucientes Ruiz A, Pato Cour E, Freites Nuñez D, Jover Jover J, Abasolo Alcazar l, León L. Discontinuation of Disease Modifying Drugs Due to Inefficacy in Patients with Incident Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/discontinuation-of-disease-modifying-drugs-due-to-inefficacy-in-patients-with-incident-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-of-disease-modifying-drugs-due-to-inefficacy-in-patients-with-incident-rheumatoid-arthritis/