Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Biological DMARD (bDMARD) concomitant with methotrexate (MTX) has made great progress in the treatment of rheumatoid arthritis (RA) in these decades. There are various issues relating to safety, efficacy and economics in clinical practice during long-term therapy. Some patients discontinue MTX due to toxicity including gastrointestinal (GI) disorders. Thus, de-escalation of MTX during treatment with bDMARD while maintaining a favourable disease activity state may be beneficial treatment option from the perspective of reducing adverse events. The efficacy of tocilizumab (TCZ) has been demonstrated in monotherapy as well as with concomitant MTX, opening up the possibility of MTX discontinuation in these patients if disease control can be maintained.
This study aimed to evaluate the efficacy and safety of MTX discontinuation in RA patients with sustained low disease activity undergoing combination therapy with TCZ plus MTX.
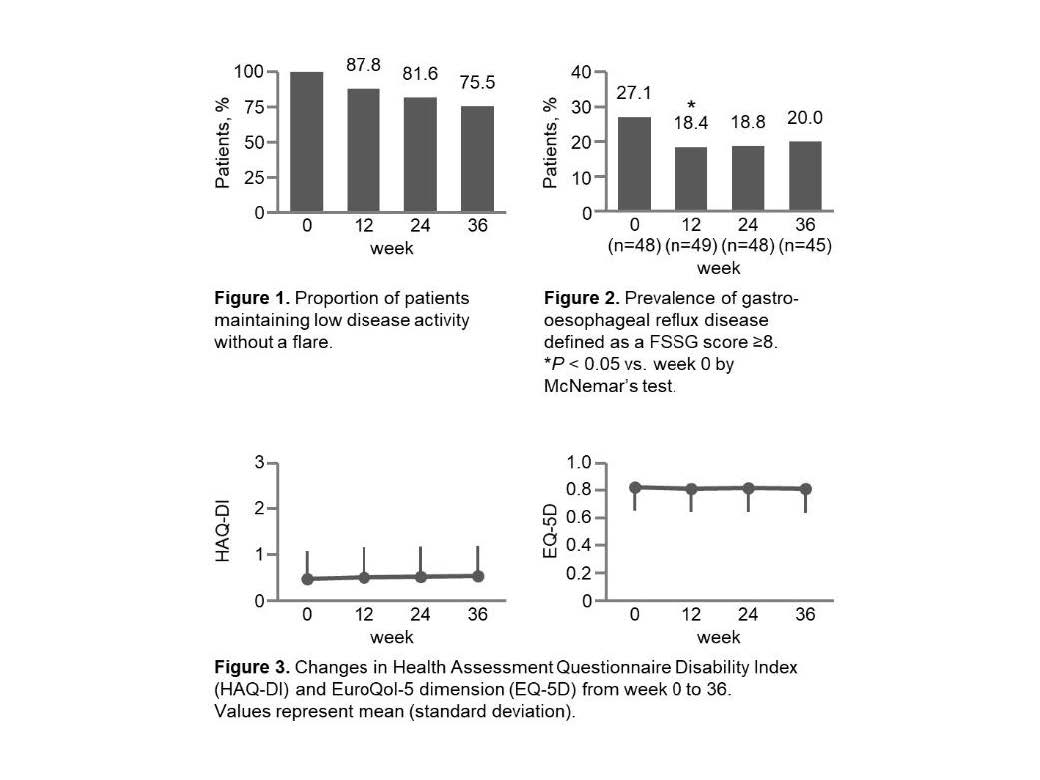
Methods: This multicentre, open-label, uncontrolled, prospective 64-week study included RA patients maintaining low disease activity (Clinical Disease Activity Index [CDAI] ≤10) for ≥12 weeks with TCZ plus MTX. MTX was discontinued following 12 weeks of biweekly administration while continuing TCZ therapy. The rescue treatments were performed if the CDAI score was >10 and at the discretion of the investigator and/or upon patient request. The primary endpoint was the proportion of patients maintaining low disease activity with no flare at week 36 (24 weeks after MTX discontinuation). Disease flare was defined as a CDAI score >10 or intervention with the rescue treatments for any reasons. Assuming that 80% of patients would maintain low disease activity at week 36, 43 patients were calculated as necessary to prove the clinical feasibility of discontinuing MTX at a power of ≥80% with a threshold response rate of 60%. Secondary endpoints were GI symptoms (Frequency Scale for Symptoms of Gastro-oesophageal reflux disease score), physical function (HAQ-DI), and quality of life(EQ-5D).
Results: A total of 49 patients completed 36 weeks of therapy. Table 1 shows the baseline (week 0) characteristics of patients included in the efficacy analyses. The proportions (95% CI) of patients who maintained low disease activity without a flare at weeks 12, 24, and 36 were 87.8% (75.2 – 95.4%), 81.6% (68.0 – 91.2%), and 75.5% (61.1 – 86.7%), respectively (Fig. 1). The lower limit of the 95% CI at week 36 exceeded the assumed threshold response rate of 60%, demonstrating the clinical feasibility of MTX discontinuation. The prevalence of gastro-oesophageal reflux disease, defined as a Frequency Scale for Symptoms of Gastro-oesophageal reflux disease score ≥8, significantly decreased from week 0 to 12 (27.1% to 18.4%; P = 0.025) (Fig. 2). No significant impairment were observed in the HAQ-DI and EQ-5D from week 0 to 36 (Fig. 3).
Conclusion: Discontinuation of concomitant MTX is clinically feasible for maintaining low disease activity without impairment of physical function and QOL, and may be beneficial from the perspective of reducing GI symptoms in RA patients treated with TCZ.
Conflict of interest
This study was supported by grant from Chugai Pharmaceutical CO.,LTD.
To cite this abstract in AMA style:
Kojima T, Asai S, Hanabayashi M, Hayashi M, Takahashi N, Kuwatsuka Y, Ando M, Ishiguro N. Discontinuation of Concomitant Methotrexate in Japanese Patients with Rheumatoid Arthritis Treated with Tocilizumab: An Interventional Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/discontinuation-of-concomitant-methotrexate-in-japanese-patients-with-rheumatoid-arthritis-treated-with-tocilizumab-an-interventional-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-of-concomitant-methotrexate-in-japanese-patients-with-rheumatoid-arthritis-treated-with-tocilizumab-an-interventional-study/