Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: There is a well-known risk of developing adverse drug reactions (ADR) in rheumatic patients due, mainly, to the use of Disease Modifying Drugs (DMARD). In the last two decades, the use of biological DMARDs (bDMARDs) has increased extensively but our knowledge of the ADR to these drugs outside clinical trials setting and in the long term has not at the same rate; especially those that require drug discontinuation. Purpose. To describe the incidence and characteristics of ADR to bDMARD, as well as the factors associated to their discontinuation in an inception cohort of patients with RA.
Methods: We conducted an observational longitudinal retrospective study. Patients: all recent onset RA diagnosed between January 1st 2007 and December 31st 2015 followed in outpatient clinic at Hospital Clinico San Carlos until January 1st 2022, which used any bDMARD ≥ 3 months, were included. Primary outcome: development of an ADR that required drug discontinuation (moderate: discontinuation drug, severe: hospitalization or death of the patient due to the ADR). Covariables: sociodemographic, clinical and treatment. Statistical analysis: incidence rates of discontinuation (IR) per 100 patient-years were estimated using survival techniques with their respective 95% confidence interval [CI]. A multivariate Cox regression analysis was used to evaluate the risk factors of discontinuation due to ADR. Results were expressed as HR with their 95% CI.
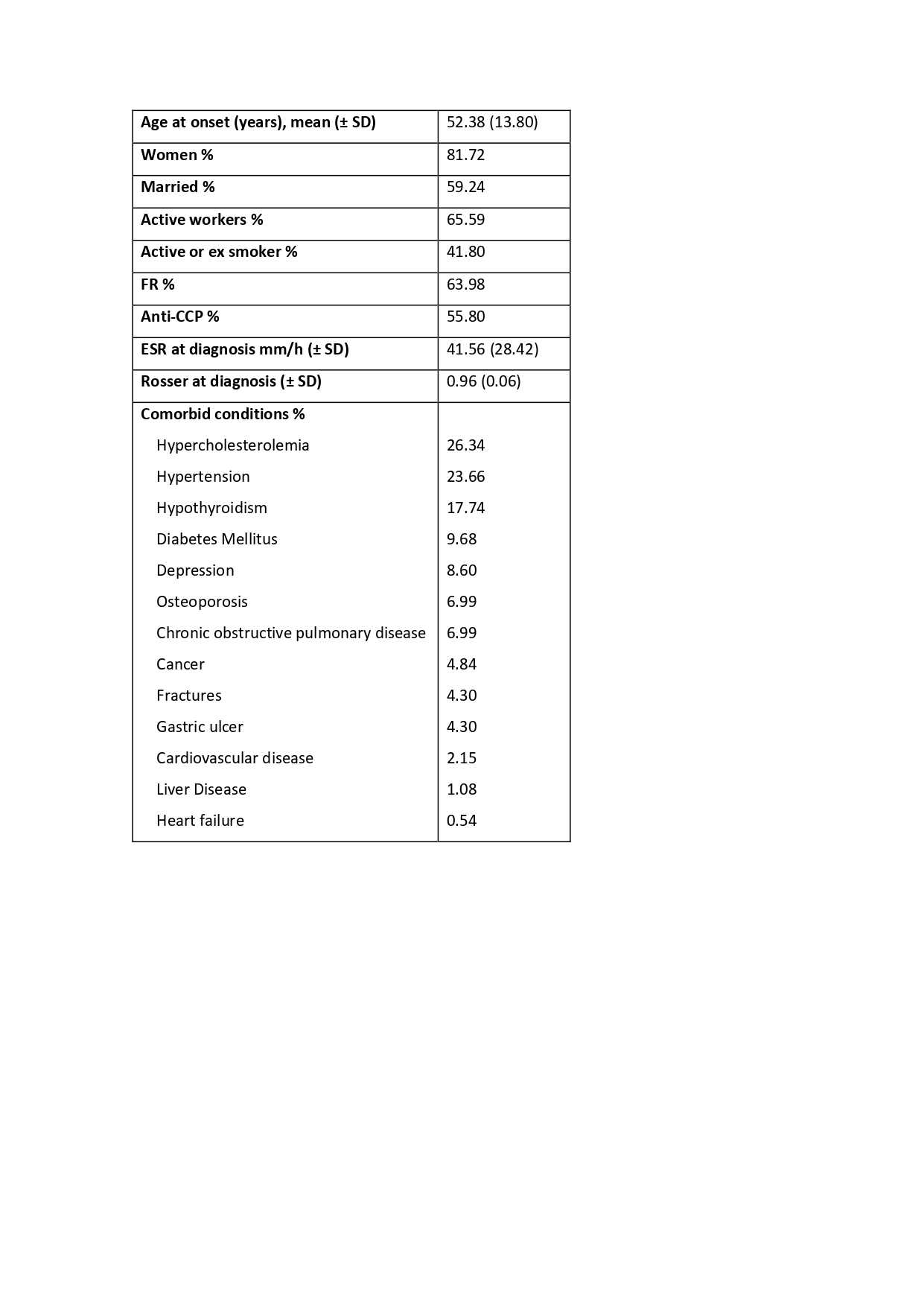
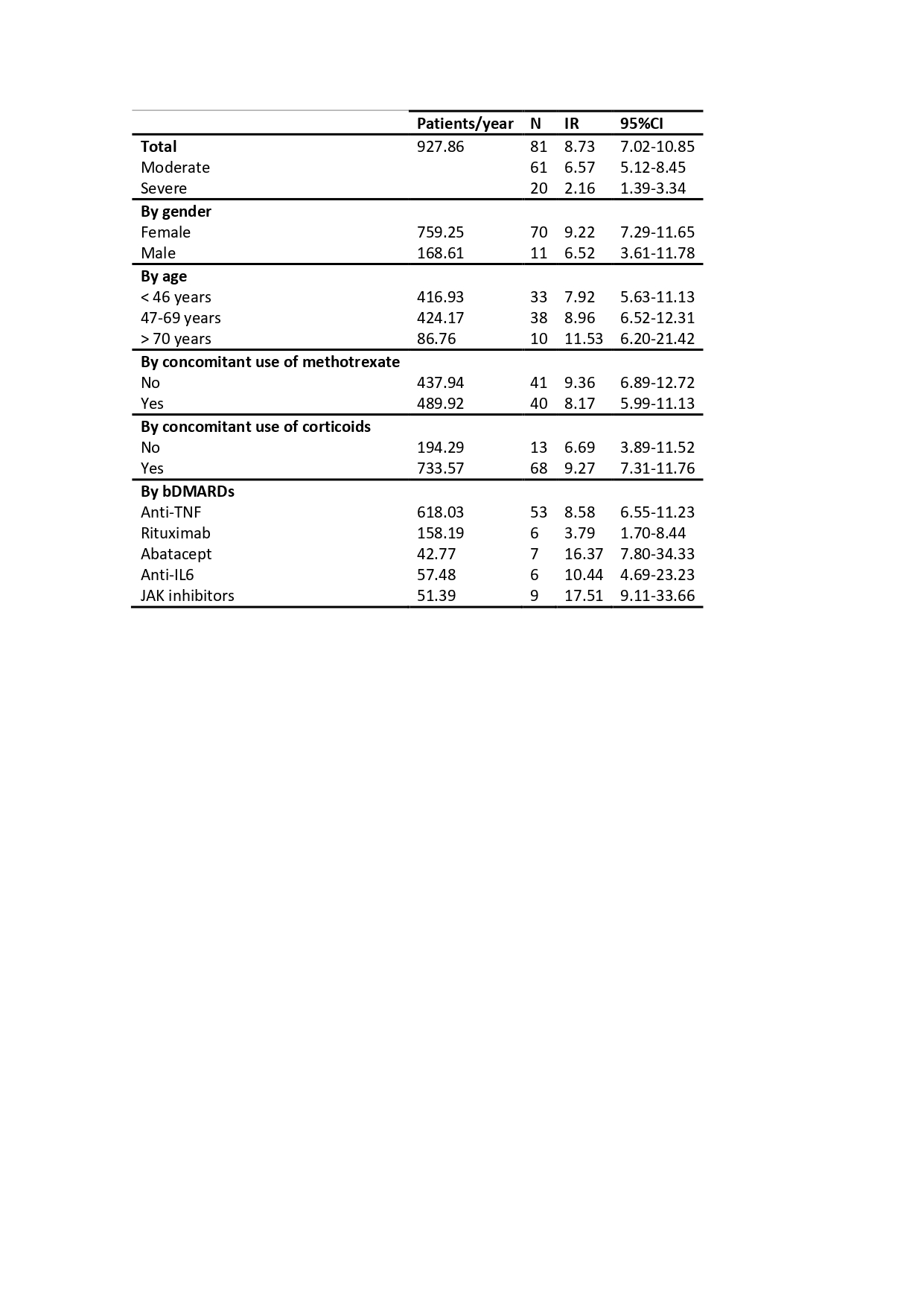
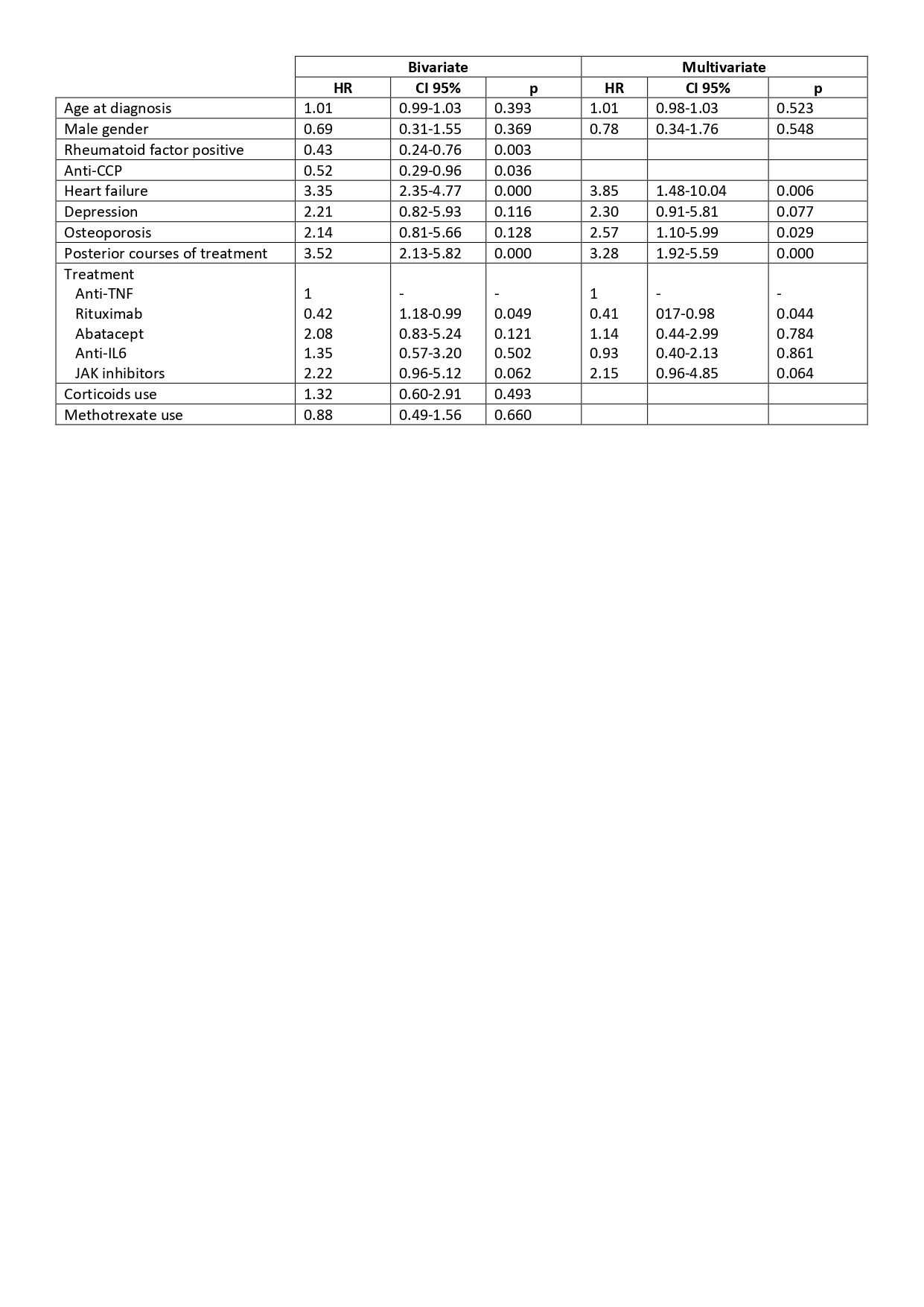
Results: 186 patients were included (927.86 patient-years), 81.72% were women with a mean age at diagnosis of 52.38±13.80. Characteristics at baseline are shown in table 1. They received 347 courses of bDMARD treatment of which 81 were discontinued due to ADR (11.50%; IR 8.73 [7.02-10.85]). 75.30% of the ADR were moderate (IR 6.57 [5.12-8.45]) and 24.69% severe (IR 2.16 [1.39-3.34]). Infection was the most frequent cause of ADR (n=28, 35.44%), followed by allergic reactions (n=16, 20.25%); 2 patients died due to an ADR. Incidence rates are shown in table 2. After performing bivariate and multivariate analysis (table 3) we found that the risk of ADR was higher in patients with heart failure (HR 3.85 [1.48-10.04]) or osteoporosis (HR 2.57 [1.10-5.99]) at diagnosis and in posterior courses of treatment (HR 3.28 [1.92-5.59]); there was a tendency of higher risk to develop ADR with the use of JAK inhibitors (HR 2.15 [0.96-4.85]) compared to anti-TNF, but it did not reach statistical significance. Rituximab had lower risk (HR 0.41 [0.17-0.98]) for the development of ADR compared to anti-TNF.
Conclusion: Discontinuations of bDMARDs in clinical practice due to ADR are common (13.06%) with an estimated IR of 8.73%, most of them moderate. Infection was the main cause of ADR with only 2 deaths registered due to an ADR during follow-up. Higher incidences of ADR to bDMARD were found in the female sex, older patients, concomitant use of corticoids as well as with some bDMARDs (JAKi and Abatacept). We should pay special attention regarding the development of ADR to patients with certain comorbidities and those using posterior courses of treatment, meanwhile Rituximab seems a safer option of treatment compared to anti-TNF.
To cite this abstract in AMA style:
Rosales-Rosado Z, Rodriguez Laguna M, lajas Petisco C, De Miguel C, Abasolo l. Discontinuation of Biological Disease Modifying Drugs Due to Adverse Drug Reactions in an Inception Rheumatoid Arthritis Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/discontinuation-of-biological-disease-modifying-drugs-due-to-adverse-drug-reactions-in-an-inception-rheumatoid-arthritis-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-of-biological-disease-modifying-drugs-due-to-adverse-drug-reactions-in-an-inception-rheumatoid-arthritis-cohort/