Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Patient Outcomes, Preferences, & Attitudes (0789–0794)
Session Type: Abstract Session
Session Time: 1:30PM-1:45PM
Background/Purpose: We previously described the top-line results of our study and the high correlations between virtual SLE disease activity measures (DAM) and those obtained during face-to-face (F2F) visits. Here we describe the more granular data on disagreements between telemedicine (TM) and F2F DAM across 2 visits in order to better understand the reasons for these discrepancies and improve TM assessments of SLE activity.
Methods: This was an observational, longitudinal study of SLE patients from 4 academic lupus centers serving diverse populations supported by a grant from the US DoD. Each study participant was evaluated at a baseline and follow-up visit as dictated by usual care. Virtual physical exam guidelines were established and relied upon clinician-directed patient self-examination. At each visit, participants were evaluated by the same provider via a videoconference-based TM and then a F2F encounter. SLE DAM were completed after the TM and the F2F encounters. Disagreements in DAM domains between the encounters were noted and verified with the evaluator, as were changes in treatment plan. Tandem provider and patient feedback tools for encounters probed satisfaction, comfort, and problematic evaluation.
Results: 163 racially and ethnically diverse participants were enrolled and completed the first visit, 145 completed the follow-up visit as previously described (Table 1). Briefly, while intraclass correlation coefficients (ICC) showed high correlation between TM and F2F (0.877-0.998), paired T-tests showed differences for SLEDAI (mean difference -0.19 +/-1.33, p=0.011) and CLASI activity (mean differences -0.18 +/-0.76, p < 0.001); the main drivers were difficulty with virtual arthritis and rash evaluations. For the 145 baseline/follow-up pair visits, there were 63 disagreements across all DA instruments: 25 musculoskeletal (10 overestimating [OE], 15 underestimating [UE] DA), 25 mucocutaneous (7 OE, 18 UE), 3 cardiorespiratory [3 UE], 3 vasculitis [3 UE], 6 constitutional [3 OE/3 UE], 1 ophthalmologic [1 UE] (Fig. 1). These resulted in inconsistencies in SLEDAI 38 (pleurisy-1, arthritis-14, ulcers-5, rash-9, alopecia-6, vasculitis-3) and BILAG 36 (constitutional-6, mucocutaneous-14, musculoskeletal-14, cardiorespiratory-1, ophthalmologic-1). Sometimes, arthritis not recorded on the virtual SLEDAI, upon F2F evaluation swollen and tender joints were elicited by the clinician and resulted in SLEDAI scoring of arthritis, but arthralgia/mild arthritis was recorded on the BILAG. Following the F2F visit 52.9% clinicians were highly confident that their virtual DA assessment was accurate, 41.0% confident/moderately confident, 4.4% minimally confident, and 1.7% not confident at all.
Conclusion: These data discuss lack of agreement for the mucocutaneous and arthritis findings, despite high overall correlations between virtual and F2F DA. TM tended to slightly underestimate DA. These data emphasize the need to further refine virtual DA assessments to provide improved agreement between TM and F2F evaluations and allow for increased adoption of telemedicine in SLE clinical care and clinical trials.
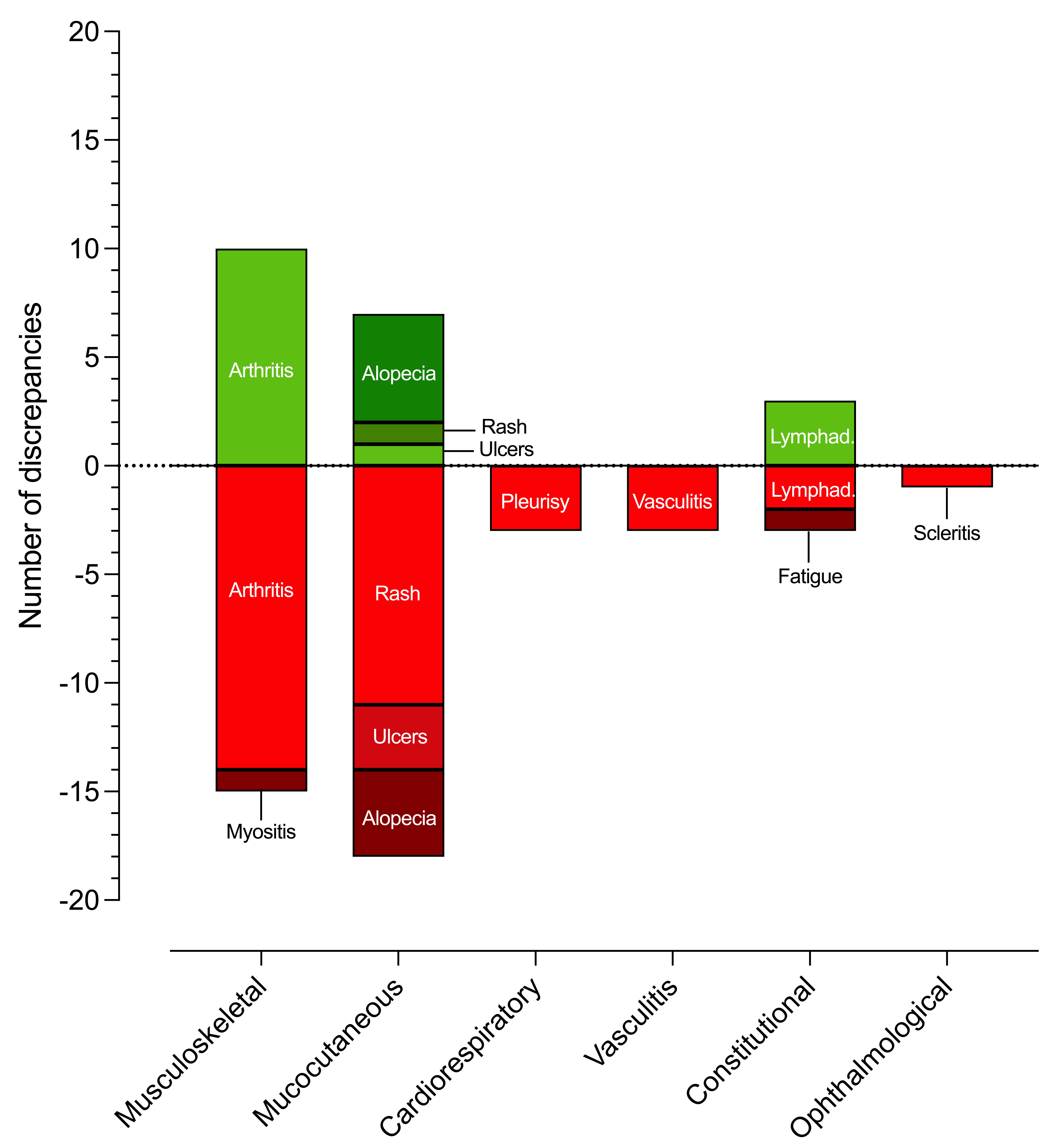 Table 1. Baseline demographic and clinical characteristics of the participants.
Table 1. Baseline demographic and clinical characteristics of the participants.
.jpg) Figure 1. Discrepancies in disease activity scores between telemedicine and face-to-face visits.
Figure 1. Discrepancies in disease activity scores between telemedicine and face-to-face visits.
To cite this abstract in AMA style:
Khalili L, Aranow C, Kim M, Kamen D, Arriens C, Nordmann-Gomes A, Souvignier M, Tang W, Suh S, Dall'Era M, mackay M, Askanase A. Disagreements in Disease Activity Measures in an Evaluation of SLE Outcome Measures in Telemedicine [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/disagreements-in-disease-activity-measures-in-an-evaluation-of-sle-outcome-measures-in-telemedicine/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disagreements-in-disease-activity-measures-in-an-evaluation-of-sle-outcome-measures-in-telemedicine/
