Session Information
Date: Monday, November 18, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Anti-synthetase syndrome (ASSD) is a rare systemic autoimmune rheumatic disease with significant heterogeneity. The Classification Criteria for Anti-Synthetase Syndrome (CLASS) project is an international collaborative study to develop and validate classification criteria for ASSD. We performed an unsupervised cluster analysis of ASSD patients registered in the CLASS database to better understand the clinical heterogeneity of this disease entity.
Methods: We performed a multiple corresponding analysis followed by hierarchical clustering to aggregate ASSD patients into clinically distinct clusters, using 15 key variables covering the clinical manifestations of ASSD. Clinical characteristics and overall survival of patients were compared between the clusters. A simple classification tree was developed using the classification and regression tree (CART) analysis within the randomly selected derivation dataset (70% of the entire dataset). The performance of the classification tree was assessed in the internal validation dataset (the remaining 30% of the dataset). As a sensitivity analysis, we repeated the cluster analysis, excluding anti-synthetase and anti-Ro52 antibodies from the variables used for clustering.
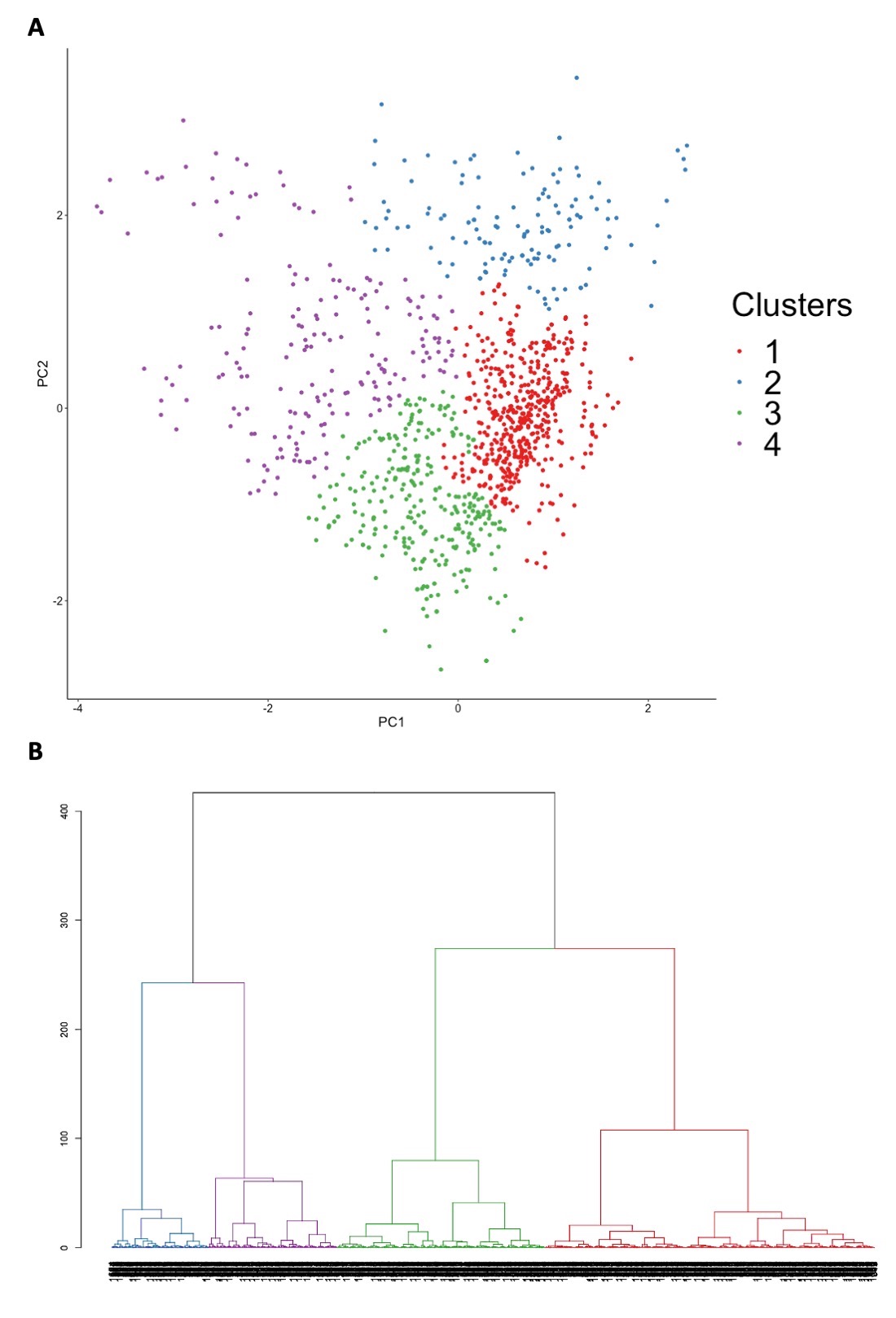
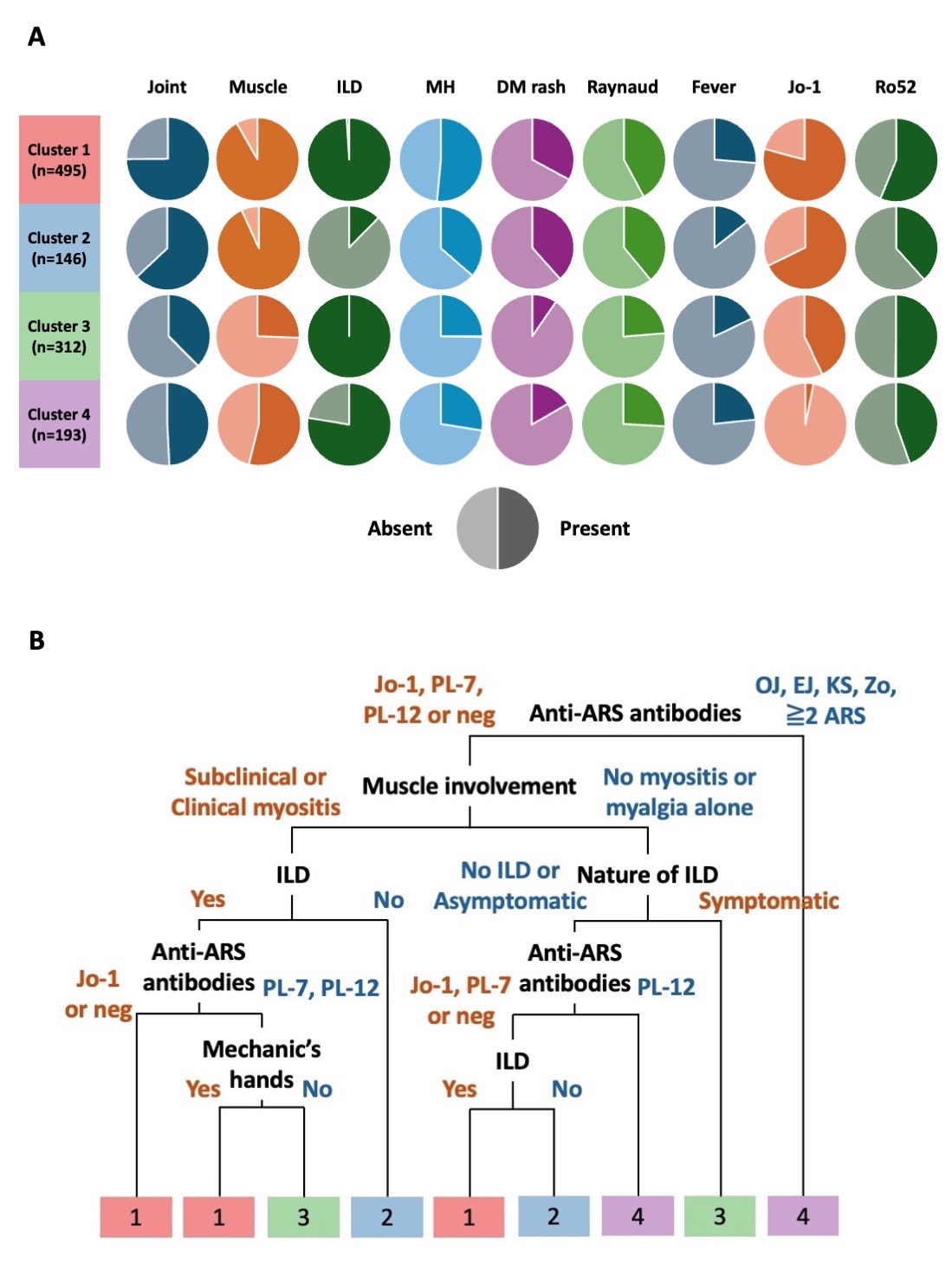
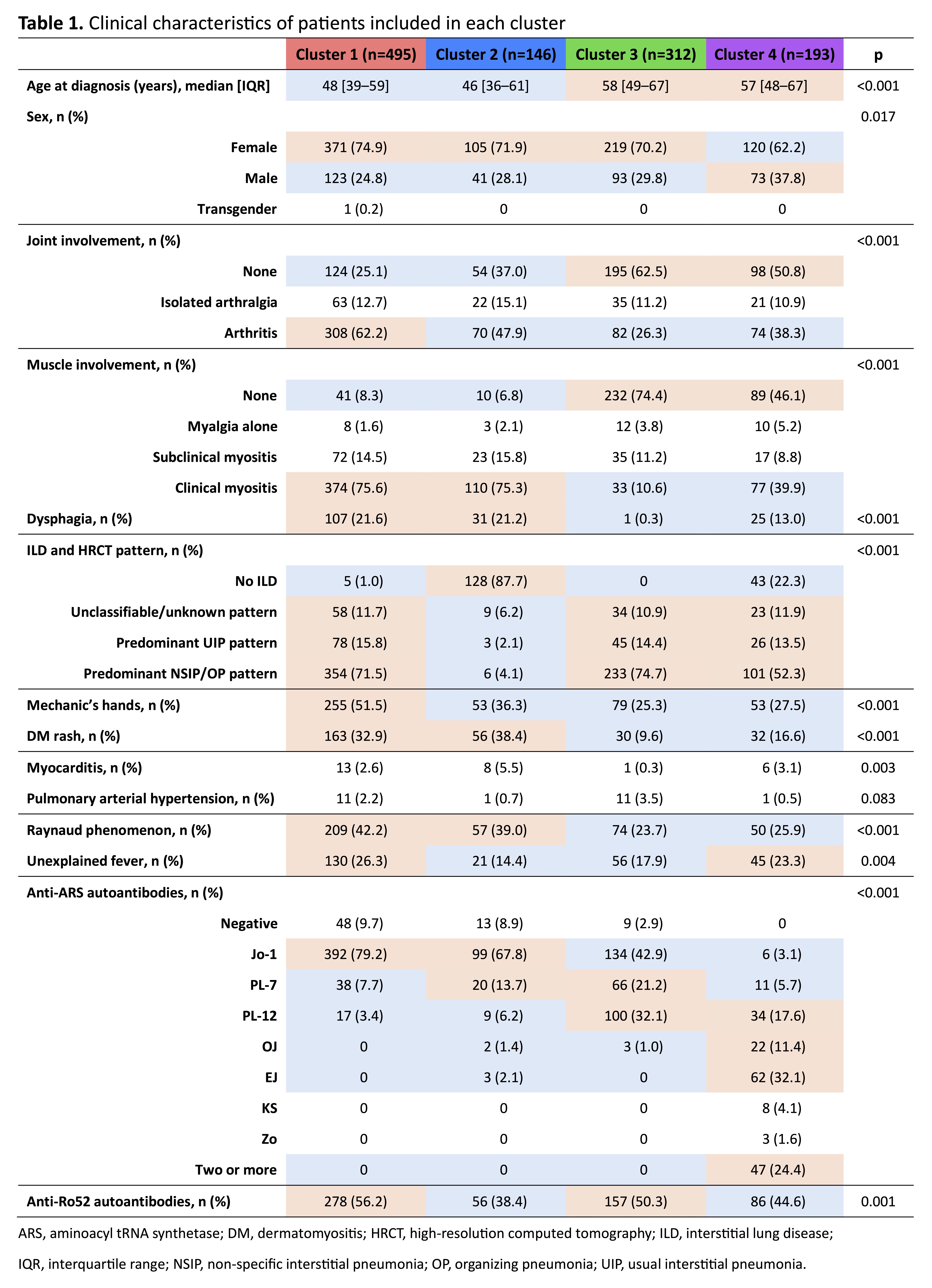
Results: Among 1863 ASSD patients, 717 were excluded due to missing data in the variables necessary for clustering, and 1146 were subjected to cluster analysis (Figure 1A and 1B). The characteristics of eligible patients and those excluded were largely similar. We identified four clinically distinct clusters (Table 1 and Figure 2A). Cluster 1 (n=495) was characterized by a higher prevalence of arthritis, myositis, ILD, mechanic’s hands, dermatomyositis (DM) rash, Raynaud phenomenon, unexplained fever, and anti-Jo-1 antibodies. Cluster 2 (n=146) corresponded to patients without ILD, while associated with a high prevalence of myositis, DM rash, Raynaud phenomenon, and anti-Jo-1 or PL-7 antibodies. Cluster 3 (n=312) was an ILD-predominant cluster, associated with a lower prevalence of joint, muscle, and skin involvements. Anti-PL-7 and PL-12 antibodies were more prevalent in this cluster. Cluster 4 (n=193) was also an ILD-predominant cluster, associated with male sex and a higher prevalence of non-Jo-1 anti-synthetase antibodies, namely anti-PL-12, EJ, and OJ antibodies. There was no significant difference in the overall survival of patients between the clusters. CART analysis within the derivation dataset constructed a classification tree comprising anti-synthetase antibodies, muscle involvement, ILD, and mechanic’s hands (Figure 2B), which successfully positioned 82.8% of patients in the internal validation dataset into the correct clusters. In the sensitivity analysis excluding autoantibodies from the variables for clustering, five clusters (Clusters S1–S5) were identified. The characteristics of Clusters S1, S4, and S5 were consistent with those of Clusters 1, 3, and 2, respectively.
Conclusion: Unsupervised clustering in the CLASS database identified four clinically distinct subgroups in patients with ASSD. Our results provide insight into the ongoing development of classification criteria for ASSD.
To cite this abstract in AMA style:
Yoshida A, Bauer Ventura I, Dourado E, Zanframundo G, Faghihi Kashani S, Loganathan A, Rivero Gallegos D, Bozan F, sambataro G, Bae S, Trallero-Araguás E, Mammen A, Scire C, Montecucco C, Oddis C, Fiorentino D, Bonella F, Miller F, Notarnicola A, Schmidt J, Rojas-Serrano J, Hudson m, Kuwana M, Gonzalez-Gay M, McHugh N, J Corte T, J Doyle T, Werth V, Aggarwal R, Cavagna L. Different Clinical Phenotypes of Patients with Anti-synthetase Syndrome: Unsupervised Cluster Analysis in the CLASS Database [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/different-clinical-phenotypes-of-patients-with-anti-synthetase-syndrome-unsupervised-cluster-analysis-in-the-class-database/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/different-clinical-phenotypes-of-patients-with-anti-synthetase-syndrome-unsupervised-cluster-analysis-in-the-class-database/