Session Information
Date: Monday, November 8, 2021
Title: RA – Treatments Poster II: PROs, Biomarkers, & Systemic Inflammation (1223–1256)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Patients with RA in remission (REM) may experience disease-worsening (DW) following therapy change or withdrawal. The change in patient-reported outcomes (PROs) after therapy withdrawal has not been well described. This analysis examined PRO changes in RA patients in REM at baseline from a clinical trial enacting medication withdrawal.
Methods: SEAM-RA was a phase 3, multicenter, randomized withdrawal, double-blind controlled study in patients with RA on methotrexate (MTX) + etanercept (ETN) (combo) who were in REM (Simplified Disease Activity Index [SDAI] ≤3.3). After randomization to either MTX or ETN withdrawal, patients who experienced an SDAI score >11 at any time following randomization or SDAI >3.3 and ≤11 during 2 consecutive visits ≥2 weeks apart or on ≥3 separate visits were considered to have DW. Patients with DW resumed or continued MTX + ETN (rescue therapy). Patient Global Assessment of Disease Activity (PtGA), Patient Assessment of Joint Pain (PtJP), Health Assessment Questionnaire-Disability Index (HAQ-DI), and the 36-item Short-Form Health Survey (SF-36) were administered at baseline and Weeks 24 and 48 and also at Weeks 12 and 36 for PtGA and PtJP. Over the 48-week period, PROs were evaluated by a change (worsening or improvement) greater than the minimal clinically important difference (MCID) for the instrument/domain. We present cumulative data for patients with PRO change ≥MCID from baseline. Among these patients experiencing DW, we also present improvement after time of worsening.
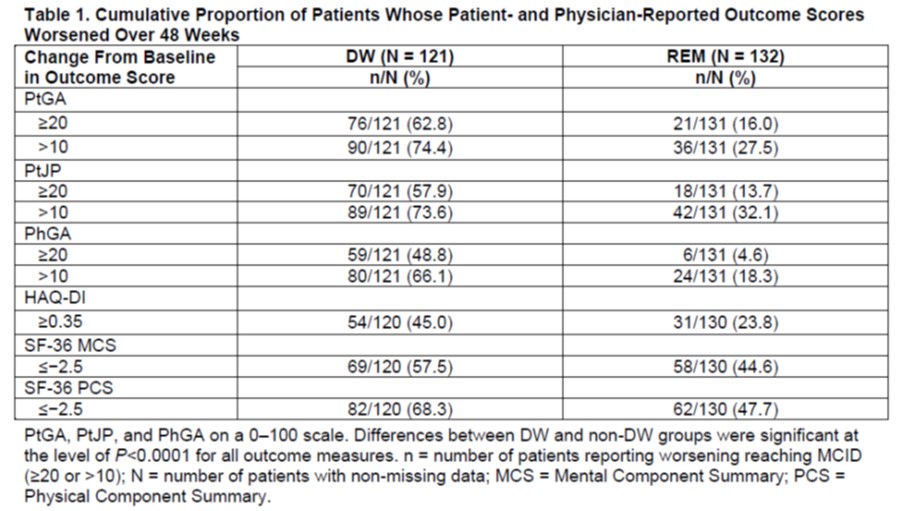
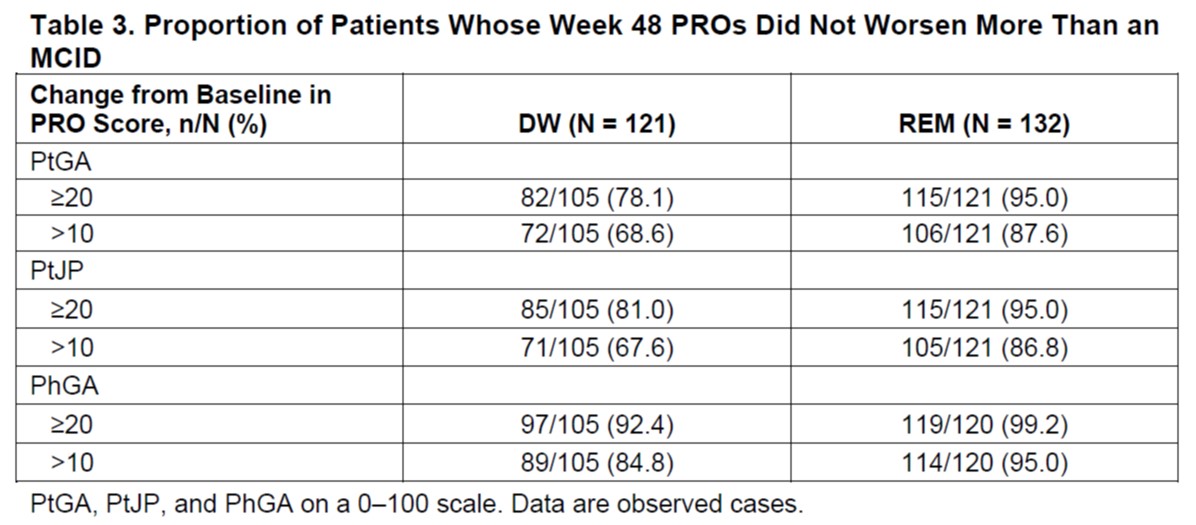
Results: Of 253 enrolled patients, 132 maintained REM and 121 experienced DW. A greater proportion of DW than REM patients had worse PtGA scores at a ≥20-point change from baseline (62.8% vs 16.0%) and at a >10-point change from baseline (74.4% vs 27.5%) (Table 1). This pattern also was observed for PtJP and Physician Global Assessment of Disease Activity (PhGA) (Table 1). Median time to PtGA worsening >MCID ( >10) was 169 days in DW patients and was shorter than median time to overall DW (198 days in the MTX group, not evaluable due to cumulative probability of DW always < 50% in the ETN and combo groups). A higher percentage of patients in DW vs REM deteriorated >MCID on the HAQ-DI (≥0.35) (45.0% vs 23.8%), SF-36 Mental Component Summary (≤−2.5) (57.5% vs 44.6%), and SF-36 Physical Component Summary (≤−2.5) (68.3% vs 47.7%) scores (Table 1). Among DW patients with a ≥20-point change from baseline in PtGA and PtJP, a greater proportion of those who received rescue therapy improved their scores than those who did not receive rescue therapy (72.6% vs 7.1% and 82.5 vs 0.0%, respectively) (Table 2). At Week 48, a higher proportion of REM vs DW patients were closer to their baseline values (< MCID) for PtGA (95.0% vs 78.1%) (Table 3); similar trends were observed for PtJP and PhGA.
Conclusion: Patients who experience a loss of REM also experience deterioration in their patient-reported global health assessment and additional joint pain. The changes in patient outcomes are experienced by more DW patients than those remaining in REM. Median time to PtGA worsening more than an MCID is shorter than median time to overall DW. DW patients who receive rescue therapy can regain their outcomes, but to a lesser extent than those remaining in REM.
To cite this abstract in AMA style:
Bykerk V, Curtis J, Emery P, Haraoui B, Karis E, Kricorian G, Collier D, Yen P, Stolshek B. Differences in Patient-Reported Outcomes Between Those Who Stay in Remission and Those Who Have Disease-Worsening Following Therapy Withdrawal in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/differences-in-patient-reported-outcomes-between-those-who-stay-in-remission-and-those-who-have-disease-worsening-following-therapy-withdrawal-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/differences-in-patient-reported-outcomes-between-those-who-stay-in-remission-and-those-who-have-disease-worsening-following-therapy-withdrawal-in-patients-with-rheumatoid-arthritis/