Session Information
Session Type: Abstract Session
Session Time: 12:15PM-12:30PM
Background/Purpose: The Dietary Interventions in Psoriatic Arthritis (DIPSA) study is a multicentre randomized controlled trial (RCT) designed to evaluate the efficacy of two personalized dietary interventions in improving clinical outcomes in patients with active psoriatic arthritis (PsA), compared to standard of care.
Methods: Adults with moderately active PsA (Disease Activity index for PsA (DAPSA) >10), BMI 25–40, and stable drug therapy were recruited from 3 centres. Participants were randomized to one of 3 arms: (1) a Mediterranean (Med) diet focused on healthy food composition; (2) a low-calorie Dietary Approaches to Stop Hypertension (DASH-LC) diet targeting weight reduction; or (3) a standard-of-care control arm receiving general, non-personalized dietary advice. Interventions were delivered by registered dietitians through 2 in-person consultations and 7 structured telephone sessions. Clinical evaluations were performed at baseline, 12, and 24 weeks by rheumatologists. Patient-reported outcomes were collected from weeks 0 to 24. The primary outcome was change in DAPSA at week 12. Secondary outcomes included DAPSA change at week 24, as well as changes in pain, PROMIS fatigue, Psoriatic Arthritis Impact of Disease (PsAID), tender/swollen joint counts, anthropometric measures, and inflammatory markers. Missing data were managed using last observation carried forward and multiple imputation. Changes across study arms and from baseline were assessed using random-effects regression models.
Results: Of 92 randomized patients (Med: n=31, DASH-LC: n=30, Control: n=31), 12 withdrew post-randomization. Baseline characteristics were comparable across arms (Fig. 1). All groups experienced significant, yet modest, weight loss by week 12 (Med: –1.36 kg; DASH-LC: –2.47 kg; Control: –1.88 kg, Fig. 2A), with small additional reductions by week 24. No significant differences in weight loss were observed between arms. At week 12, significant reductions in DAPSA were observed in the DASH-LC (–4.93) and control (–4.25) groups; by week 24, all groups showed improvement (Med: –4.87; DASH-LC: –5.42; Control: –6.53, Fig. 2B), without significant between-group differences. Minimal Disease Activity (MDA) was achieved at week 12 by 38% in DASH-LC and 29% in Med versus 16% in control (p=0.23 and p=0.06, respectively), though week 24 rates were similar across groups. Improvements in pain, fatigue, PsAID, and tender joint counts were observed across all arms (Fig. 2C-G). Notably, weight loss magnitude was significantly associated with improvement in clinical outcomes—including DAPSA, pain, fatigue, PsAID, and tender joint count—independent of treatment group (Fig. 3).
Conclusion: Both personalized dietary interventions and standard dietary advice led to modest weight loss and improvements in PsA disease activity. The degree of weight loss, rather than the specific dietary strategy, was the primary determinant of clinical benefit.
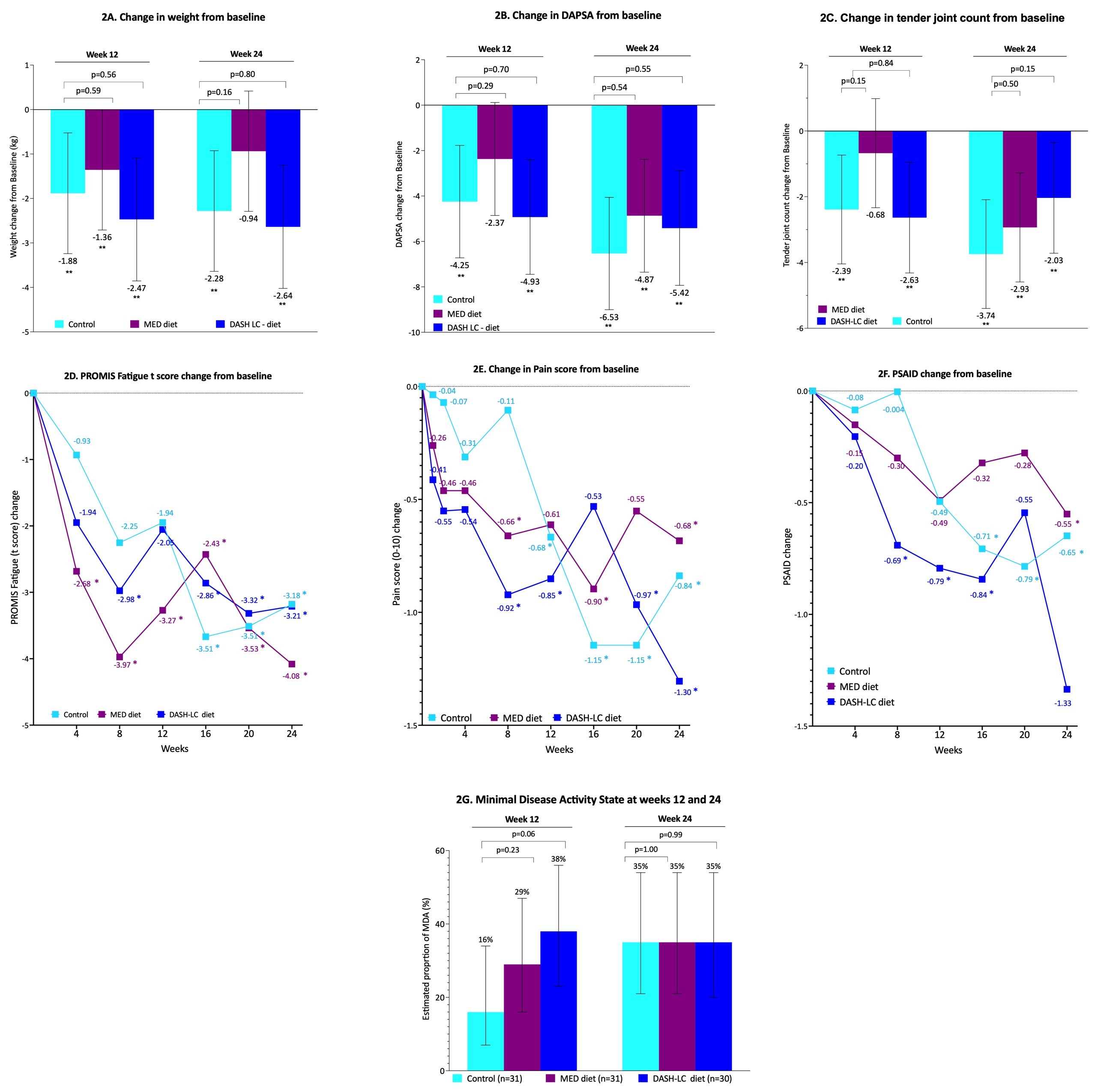 Figure 1: 1A. DIPSA study design, enrolment criteria and study procedures; 1B. Baseline characteristics by arm. BMI- Body mass area; DAPSA – Disease activity in Psoriatic Arthritis; PASI – Psoriasis Area and Severity Index
Figure 1: 1A. DIPSA study design, enrolment criteria and study procedures; 1B. Baseline characteristics by arm. BMI- Body mass area; DAPSA – Disease activity in Psoriatic Arthritis; PASI – Psoriasis Area and Severity Index
.jpg) Figure 2: Change in study outcome by study arm. 2A. Weight; 2B. DAPSA; 2C. Tender joint count; 2D. PROMIS Fatigue (t score); Pain score; 2E. PSAID; Minimal Disease Activity state. * indicates p < 0.05 compared to baseline. DAPSA - Disease activity in Psoriatic Arthritis; PSAID - Psoriatic Arthritis Impact of Disease.
Figure 2: Change in study outcome by study arm. 2A. Weight; 2B. DAPSA; 2C. Tender joint count; 2D. PROMIS Fatigue (t score); Pain score; 2E. PSAID; Minimal Disease Activity state. * indicates p < 0.05 compared to baseline. DAPSA - Disease activity in Psoriatic Arthritis; PSAID - Psoriatic Arthritis Impact of Disease.
.jpg) Figure 3. 3A-E. The correlation between study outcomes and weight change from baseline with splines; 3F-3J. The estimated mean change (95% CI) in study outcomes by the extent of weight reduction (none, < 5% and >5% reduction in body weight) for each study arm.
Figure 3. 3A-E. The correlation between study outcomes and weight change from baseline with splines; 3F-3J. The estimated mean change (95% CI) in study outcomes by the extent of weight reduction (none, < 5% and >5% reduction in body weight) for each study arm.
To cite this abstract in AMA style:
Eder L, Shahab S, Hopkins Gillespie S, Bumbulis L, Emanoilidis H, Compher C, Scher J, Gladman D, Cook R, Chandran V, Ogdie A. Dietary interventions in Psoriatic Arthritis: A Randomized controlled clinical trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/dietary-interventions-in-psoriatic-arthritis-a-randomized-controlled-clinical-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dietary-interventions-in-psoriatic-arthritis-a-randomized-controlled-clinical-trial/
