Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Available criteria for diagnosis or classification of hip osteoarthritis (OA) have been poorly validated. The few validation studies available all used radiographic features of hip OA as reference standard, despite the clinical importance of hip symptoms. Therefore, the current study aimed to assess the diagnostic performance of the clinical ACR criteria and the NICE criteria against expert-based diagnosis of clinically relevant hip OA.
Methods: The CHECK study enrolled Dutch individuals aged 45-65 years with pain/stiffness in the knees and/or hips, prior to or within 6 months after their first consultation for these complaints with a general practitioner (GP). Individuals were excluded if their complaints were due to other causes than OA. All participants were assessed at baseline and after 2, 5, 8, and 10 years, with validated questionnaires (patient characteristics and knee/hip symptoms), physical examination (joint range of motion, pain at passive motion), and radiography (Kellgren & Lawrence [KL] grading). For the current study, all baseline symptomatic hips with follow-up data available were selected for analyses.
In pairs and based on the clinical data obtained at year 5 (T5) through year 10 (T10), 24 clinical experts (13 GPs, 6 rheumatologists, and 5 orthopedists) determined the presence of ‘clinically relevant hip OA’ over the follow-up period. Experts relied on their clinical expertise for diagnosis, as no definition for ‘clinically relevant hip OA’ was provided. First, experts evaluated all hips individually, after which a consensus meeting was held to discuss cases with conflicting diagnoses.
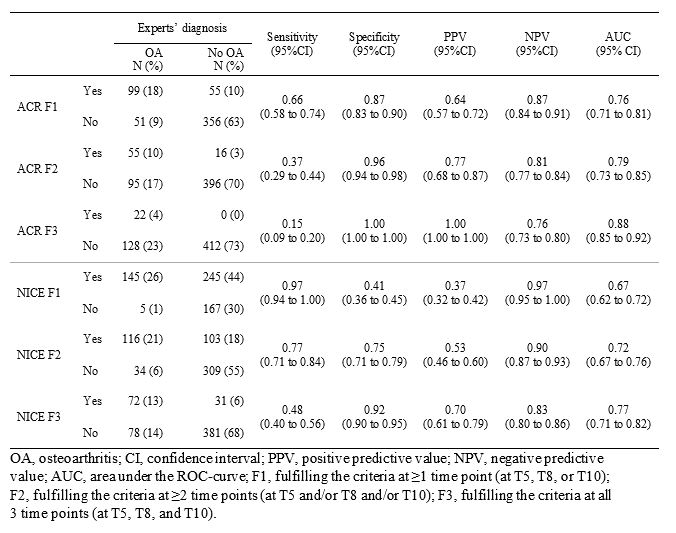
At each time point separately, the fulfilment of the clinical ACR criteria (hip pain + internal rotation < 15° + flexion of the hip ≤115°; or hip pain + internal rotation ≥15°, pain at internal rotation, stiffness ≤60 min + age >50 years) and the NICE criteria (activity related hip pain + no morning stiffness or < 30 minutes + aged ≥45 years) was determined for each hip. Next, with the expert-based diagnosis of ‘clinically relevant hip OA’ (present/absent) as reference standard, test characteristics were determined for each set of criteria in 3 definitions of a positive test, i.e. meeting a set of criteria at one, two, or at all three time points.
Results: In total, 562 hips were available for analyses. At T5, mean age was 60.3 ± 5.1 years, mean BMI was 26.6 ± 4.1 kg/m2, and 83% were female. Radiographic hip OA (KL≥2) was present in 22% of hips at T5, 28% at T8, and 49% at T10. ‘Clinically relevant hip OA’ was diagnosed in 27% of all hips. Compared to the ACR criteria, more hips fulfilled the NICE criteria once, at two time points, and at all time points. The ACR criteria outperformed the NICE criteria on specificity and positive predictive value, while sensitivity and negative predictive value were highest for the NICE criteria.
Conclusion: With clinical diagnosis as reference standard, the ACR criteria appear better suited for patient selection for clinical studies, where enrollment of a homogeneous sample of patients with a high probability of having OA is prioritized, at the cost of high false-negative rates. Conversely, NICE criteria appear better suited for clinical diagnosis, where one would like to minimize false-negatives.
To cite this abstract in AMA style:
Runhaar J, Schiphof D, Wang Q, Kloppenburg M, Boers M, Bijlsma H, Bierma-Zeinstra S. Diagnostic Performance of ACR and NICE Criteria for Hip Osteoarthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/diagnostic-performance-of-acr-and-nice-criteria-for-hip-osteoarthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/diagnostic-performance-of-acr-and-nice-criteria-for-hip-osteoarthritis/