Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: The lack of classification criteria for axial disease hinders the conduct of clinical trials for juvenile spondyloarthritis (JSpA). We aimed to develop candidate classification criteria to identify a homogeneous group of JSpA and axial disease for entry into clinical studies. These criteria are not intended to capture all possible subjects, but instead most patients with shared key features of axial disease.
Methods: Clinical factors relevant for distinguishing JSpA with axial disease from other similar conditions were identified during item generation/reduction exercises and then collected on a standardized case report form for 304 cases with JSpA and suspected axial disease from 6 international centers; all had an MRI with views of the pelvis reviewed by a team of musculoskeletal imaging experts. Clinical SpA experts (N=14) reviewed each patient and rated the likelihood the case represented JSpA with axial disease on a scale of -3 to +3. 20 cases representing the full spectrum of ratings were then scored and rank-ordered. The clinical expert panel reviewed the interrater agreement of case ranking, refined the criteria definitions and domain levels, and participated in a multi-criterion decision analysis exercise to generate relative weights of the criteria. Next, 30 cases from the derivation cohort were scored and ranked using the relative weights for each domain. Experts independently determined the score or “threshold” below which they were no longer confident that a patient should be classified as having axial disease. Results of this exercise were discussed and a provisional threshold score for classification was achieved by consensus.
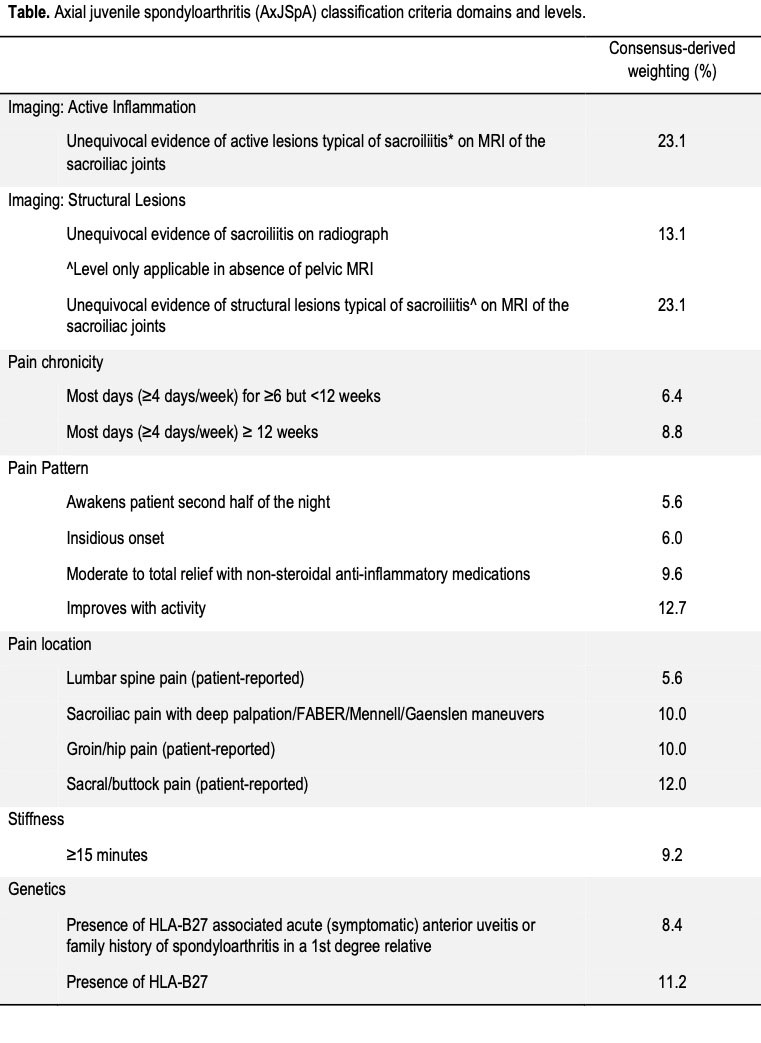
Results: The preliminary axial disease criteria for juvenile SpA included the Paediatric Rheumatology International Trials Organization (PRINTO) provisional criteria for enthesitis/spondylitis-related juvenile idiopathic arthritis (JIA) or a rheumatologist diagnosis of JSpA as entry criteria as well as additive weighted criteria for 7 domains: sacroiliac joint (SIJ) active inflammation on imaging, SIJ structural lesions on imaging, pain chronicity, pain pattern, pain location, morning stiffness, and genetics. Interrater agreement of the pre-consensus meeting case rankings was poor with a Kendall’s correlation coefficient of 0.23. The multi-criterion decision analysis was repeated for the imaging domains when initial criteria weighting was not in accordance with the expert panel’s opinion. The multi-criterion decision analysis involved a total of 58 pairwise decisions agreed upon by expert consensus. After criteria weights and additive scores were re-calculated, experts reached consensus for axial disease in juvenile SpA for all cases scoring ≥55.
Conclusion: Using an iterative process, the JSpA axial disease criteria definitions were refined, preliminary weights were generated, and a provisional threshold score for classification was determined. The most heavily weighted domains were active inflammation and structural lesions on imaging. Imaging typical of sacroiliitis was necessary, but not sufficient without any clinical criterion, to surpass the axial disease classification threshold.
To cite this abstract in AMA style:
Weiss P, Brandon T, Aggarwal A, Burgos vargas R, Colbert R, Horneff G, Joos R, Laxer R, Minden K, Ravelli A, Ruperto N, Smith J, Stoll M, Tse S, Van den bosch F, Maksymowych W, Lambert R, Biko D, Chauvin N, Francavilla M, Jaremko J, Herregods N, Kasapcopur O, YILDIZ M, Hendry A. Development, Refinement and Weighting of Candidate Criteria for Axial Disease in Juvenile Spondyloarthritis: An International Collaboration [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/development-refinement-and-weighting-of-candidate-criteria-for-axial-disease-in-juvenile-spondyloarthritis-an-international-collaboration/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-refinement-and-weighting-of-candidate-criteria-for-axial-disease-in-juvenile-spondyloarthritis-an-international-collaboration/