Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes I: Biomarkers
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Elevated IFN activity is observed in a subset of lupus subjects suggesting that those with higher levels of IRGs may benefit from interferon targeted therapies, however, to date responses by this dichotomization have been inconsistent. Tyrosine kinase 2 (TYK2) mediates cytokine pathways (eg, type I IFN, IL-12 and IL-23) linked with SLE pathogenesis. Deucravacitinib is a first-in-class, oral, selective, allosteric TYK2 inhibitor and was found to be efficacious in a phase 2 SLE trial.1 We developed a customized IFN 5-gene signature score, assessed the pharmacodynamic effects of deucravacitinib on the IFN score, and evaluated the score’s association with SLE disease activity and clinical response in the phase 2 trial (NCT03252587).
Methods: Patients were randomized equally to placebo or deucravacitinib (3 mg twice daily [BID], 6 mg BID, or 12 mg once daily [QD]). DxTerity chemical ligation-dependent probe amplification was used to measure 51 immune system–related genes from whole blood. IFN genes were selected based on distribution, correlations, hierarchical clustering, and consistency of k-means clusters. Serum proteins, blood cell subsets, and antibodies were measured by immunoassays and flow cytometry. Systemic Lupus Erythematosus Responder Index-4 (SRI[4]) and British Isles Assessment Group–based Composite Lupus Assessment (BICLA) were measured at weeks 32 and 48.
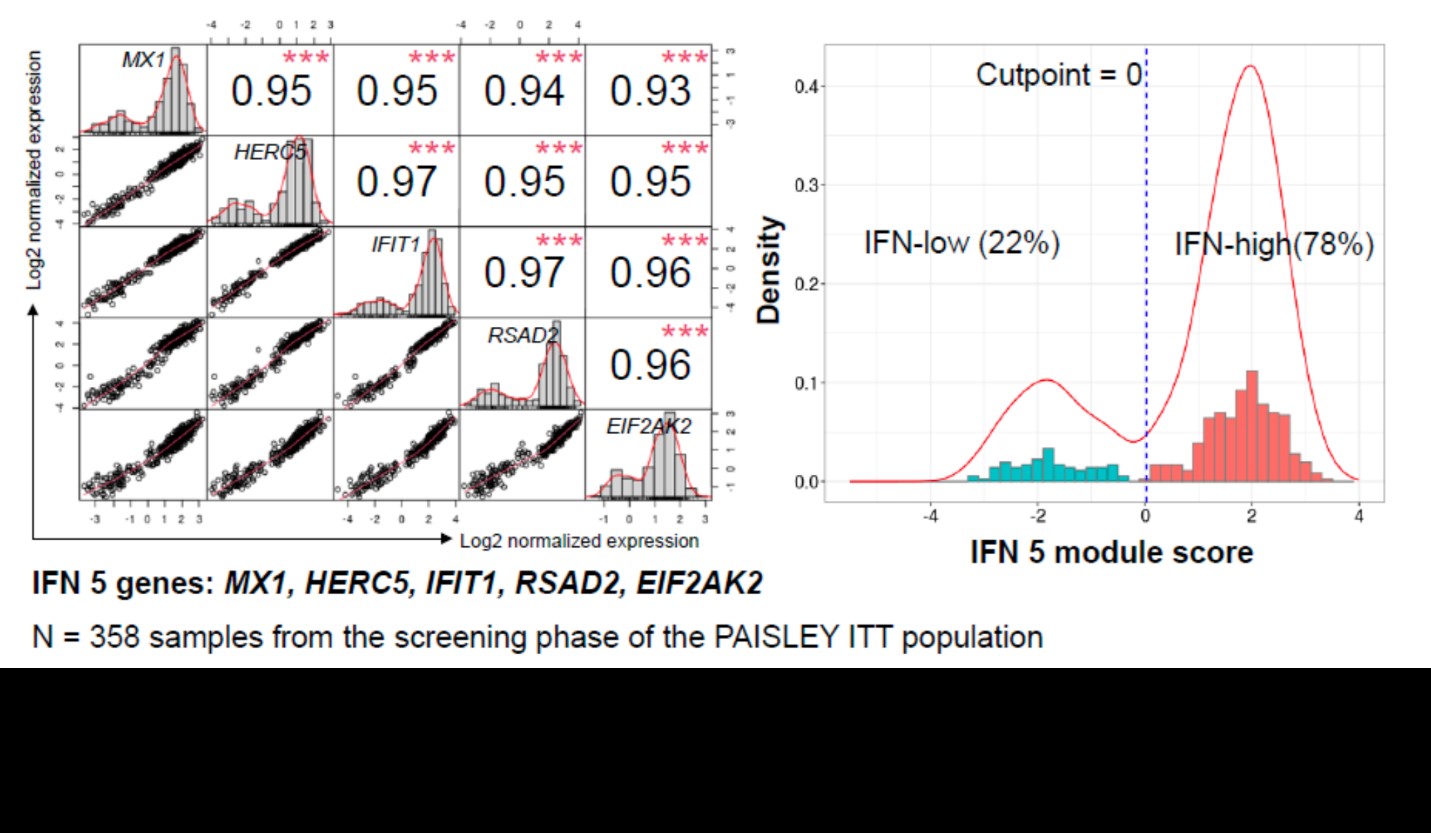
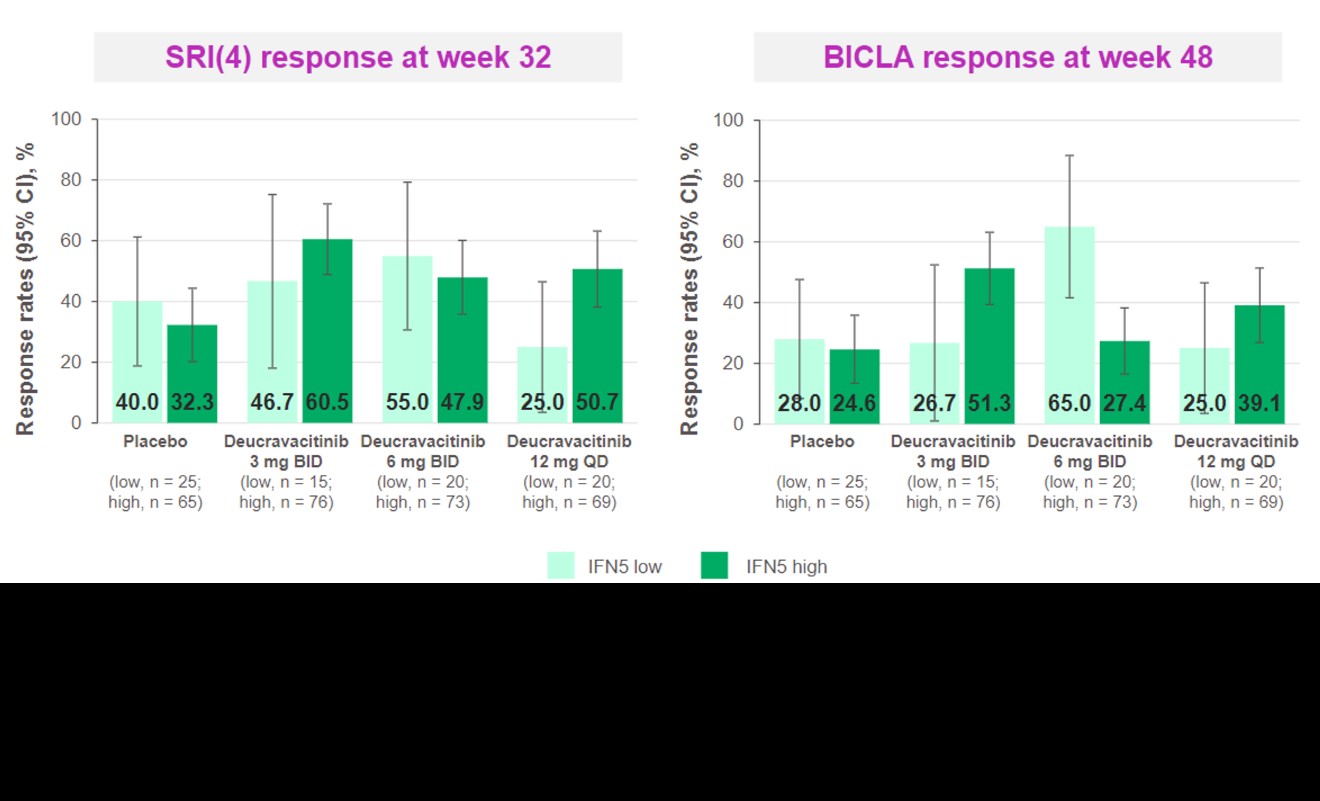
Results: An IFN 5-gene (MX1, HERC5, IFIT1, RSAD2, and EIF2AK2) signature score was identified and used to classify patients into IFN-high or IFN-low subgroups (Figure 1). Higher baseline score was associated with higher baseline SLEDAI and Cutaneous Lupus Erythematosus Disease Area and Severity Index scores, higher IFN activity biomarker (eg, IFN????, IFN????, B-cell activating factor, C-X-C motif chemokine ligand 10) and anti–double-stranded DNA levels, and lower complement and lymphocyte counts. Deucravacitinib reduced the IFN score from weeks 4 through 44 by > 50%. Patients with high IFN scores had numerically higher SRI(4) response rates at week 32 and BICLA response rates at week 48 in the 3-mg BID and 12-mg QD dose groups, but not in the 6-mg BID group, compared with patients with low IFN score (Figure 2).
Conclusion: These data support the IFN 5-gene signature score as a biomarker to classify patients with SLE into IFN-high or IFN-low subgroups; however, clinical response by IFN score was inconsistently improved. IFN-regulated gene expression performs well as a pharmacodynamic biomarker to confirm deucravacitinib mechanism of action and to aid in phase 3 dose selection.
Reference:
1. Morand E, et al. Arthritis Rheumatol 2023;75:242–252.
To cite this abstract in AMA style:
Wu C, Hu Y, Crow M, Saxena A, Arriens C, Hobar C, Coles A, Catlett I. Development of an IFN 5-Gene Signature Score to Identify IFN-high and IFN-low Subsets and as a Pharmacodynamic Biomarker for Deucravacitinib Treatment in a Phase 2 Trial in Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/development-of-an-ifn-5-gene-signature-score-to-identify-ifn-high-and-ifn-low-subsets-and-as-a-pharmacodynamic-biomarker-for-deucravacitinib-treatment-in-a-phase-2-trial-in-patients-with-systemic-lupu/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-an-ifn-5-gene-signature-score-to-identify-ifn-high-and-ifn-low-subsets-and-as-a-pharmacodynamic-biomarker-for-deucravacitinib-treatment-in-a-phase-2-trial-in-patients-with-systemic-lupu/