Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The MBDA score, based on 12 serum proteins, is a validated tool for assessing disease activity in RA patients. MBDA biomarkers may be influenced by age, sex and adiposity. The objective of this study was to develop and validate an adjusted MBDA score that accounted for these three factors, using BMI or serum leptin as proxies for adiposity.
Methods: The MBDA score as a continuous variable was adjusted to account for age, sex and a proxy for adiposity (serum leptin) using data from 325,781 RA patients for whom MBDA tests had been ordered as part of routine care. Leptin values came from the MBDA test. As an alternative to using leptin to adjust for adiposity, a cohort of 1411 patients from 5 studies/registries (BRASS, CERTAIN, InFoRM, OPERA, RACER) was used to adjust for BMI, which was not available in the larger cohort, adding this BMI adjustment to that for age/sex from the larger cohort. Both types of adjusted MBDA score use the low, moderate, and high disease activity cutpoints of the original MBDA score. The two types of adjusted MBDA score and other variables were then evaluated for the prediction of radiographic progression (RP) in the 2 cohorts with available radiographic data (OPERA and BRASS) using univariate and multivariate linear regression analyses. Rate of RP was assessed as the change in modified total Sharp score (DmTSS) per year after MBDA testing. Prediction of RP was evaluated using the original and adjusted MBDA scores and compared with conventional measures, as well as DAS28 without CRP or ESR (DAS28*).
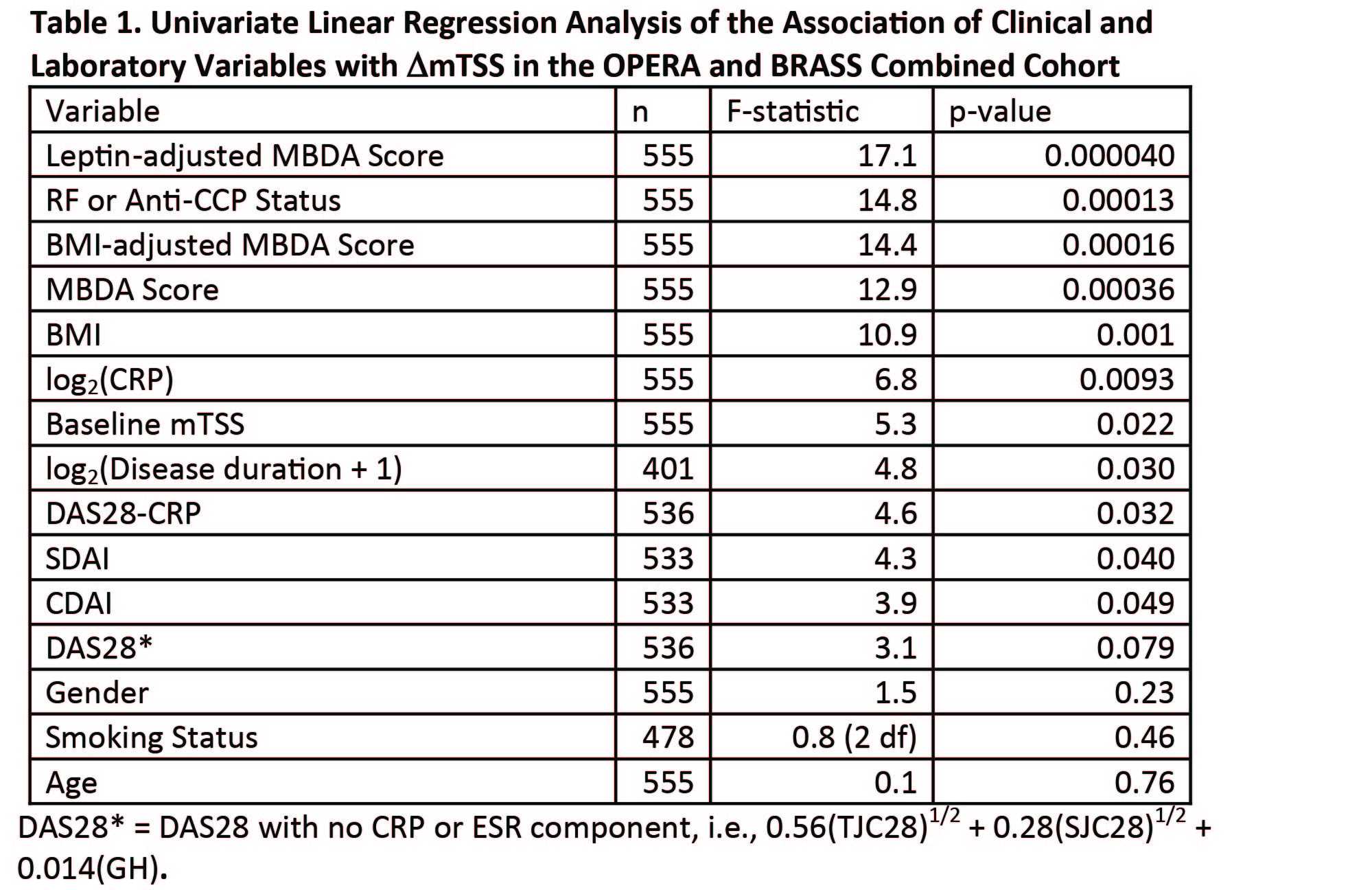
Results: The MBDA score increased with age, BMI and leptin concentration. In univariate analysis of the combined OPERA and BRASS cohorts (n = 555), the significant variables predicting DmTSS were leptin-adjusted MBDA score, seropositivity for RF or anti-CCP, BMI-adjusted MBDA score, MBDA score, BMI, CRP, baseline mTSS, disease duration, DAS28-CRP, SDAI, CDAI and DAS28* (Table 1). The leptin-adjusted and BMI-adjusted MBDA scores were the first and third most significant univariate predictors of DmTSS. To compare them directly, DAS28-CRP, MBDA score, BMI-adjusted MBDA score and leptin-adjusted MBDA score were combined in pairs in regression analyses of DmTSS; the BMI-adjusted (p = 0.0027) and leptin-adjusted MBDA score were significant (p = 0.00063) after adjusting for DAS28-CRP (p = 0.87 and 0.74, respectively) and the leptin-adjusted MBDA score was significant (p = 0.024 and 0.020, respectively) after adjusting for either the MBDA (p = 0.32) or BMI-adjusted MBDA scores (p = 0.094).
Conclusion: We developed two adjusted MBDA scores that combine molecular and biometric variables to account for age, sex, and adiposity. One of them, the leptin-adjusted MBDA score, significantly outperformed DAS28-CRP and the original MBDA score in predicting radiographic progression in RA patients. These results suggest that the leptin-adjusted MBDA score may offer improved clinical utility for the personalized management of patients with RA.
To cite this abstract in AMA style:
Curtis JR, Flake DD II, Weinblatt M, Shadick NA, Østergaard M, Lund Hetland M, Brahe CH, Hwang YG, Furst DE, Strand V, Etzel CJ, Pappas DA, Wang X, Hwang CC, Sasso EH, Gutin A, Hitraya E, Lanchbury JS. Development of an Adjusted Multi-Biomarker Disease Activity (MBDA) Score for Rheumatoid Arthritis (RA) That Accounts for Age, Sex and Adiposity, with Subsequent Evaluation of Ability to Predict Risk for Radiographic Damage [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/development-of-an-adjusted-multi-biomarker-disease-activity-mbda-score-for-rheumatoid-arthritis-ra-that-accounts-for-age-sex-and-adiposity-with-subsequent-evaluation-of-ability-to-predict-risk-f/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-an-adjusted-multi-biomarker-disease-activity-mbda-score-for-rheumatoid-arthritis-ra-that-accounts-for-age-sex-and-adiposity-with-subsequent-evaluation-of-ability-to-predict-risk-f/