Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Significant morbidity and mortality urge efforts to improve quality of care for people with systemic lupus erythematosus (SLE). Yet, none of the 25 American College of Rheumatology (ACR) quality measures is specific to SLE. As part of a collaboration between the Centers for Disease Control and Prevention and ACR, we used literature review and consensus methods to develop digital quality measures, defined by their reliance on electronic health data, in this case from the ACR RISE registry, to improve longitudinal SLE care and outcomes.
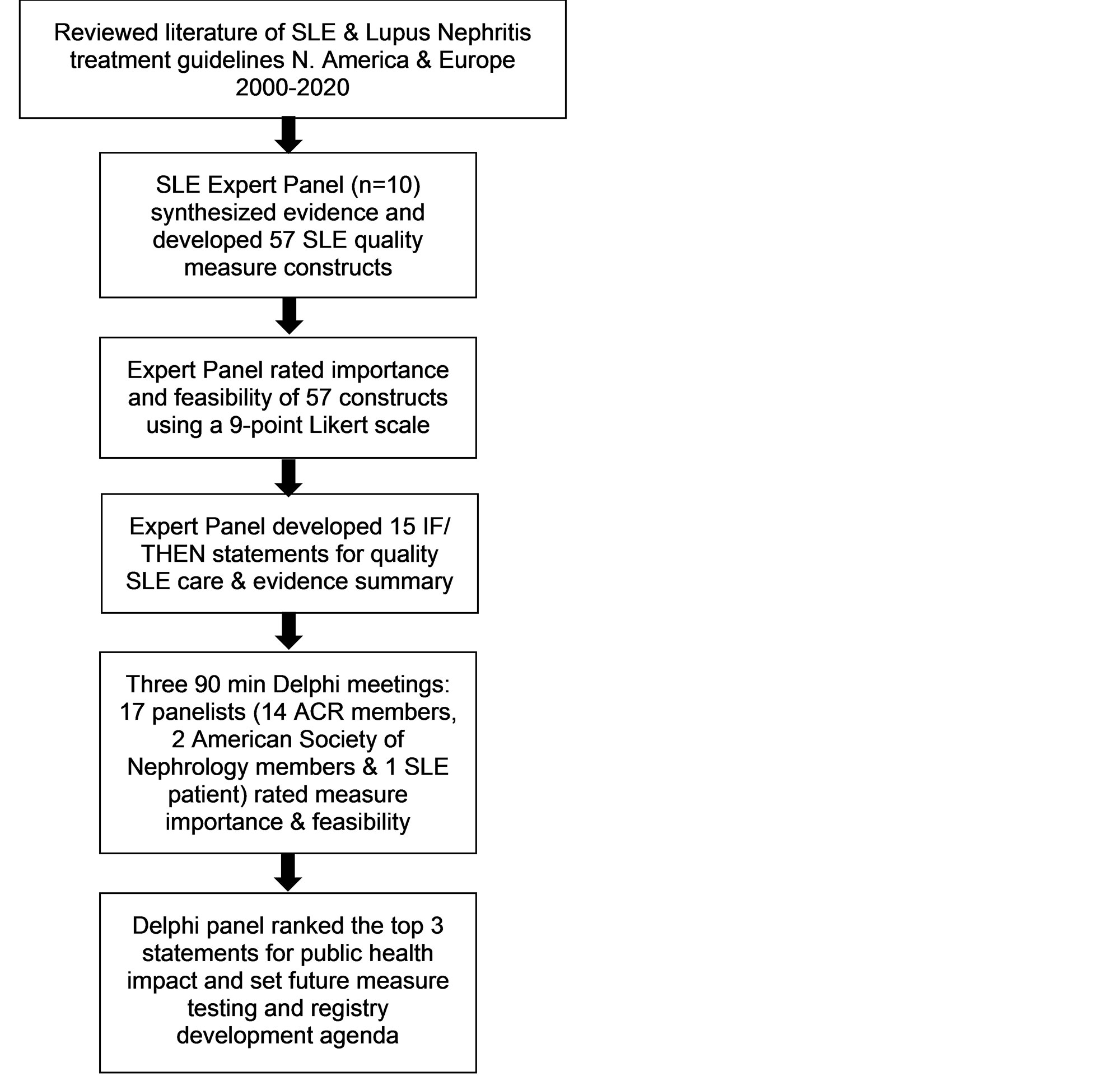
Methods: An ACR work group of 10 SLE experts reviewed literature, synthesized and ranked measure constructs, and constructed evidence-based quality measure statements for review by a 17-member multidisciplinary Delphi panel (Figure 1). A priori we planned to advance 2-3 statements to test as digital quality measures. Literature review included all N. American and European guidelines 2000-2020 focused on SLE or lupus nephritis management. Guidelines meeting inclusion criteria were reviewed to distill candidate quality measure constructs in three domains: SLE treatment, monitoring, and phenotype. After discussion, the work group ranked importance and feasibility of constructs (9-point Likert, 9=definitely important). Constructs with high importance (≥60% ratings ≥7 and ≤1 rating ≤3, after excluding 1 extreme low and high rating) were adapted as IF/THEN quality measure statements with evidence summaries. Panelists assessed each statement during three consecutive virtual Delphi sessions and voted before and after each session on importance and feasibility of each measure using 4 and 9-point Likert scales, respectively; scores were normalized to a 100-point scale. Consensus was again determined using a similar Delphi scoring schema for final votes. In a final step, the Delphi panel ranked proposed measures for potential public health impact on SLE care and outcomes.
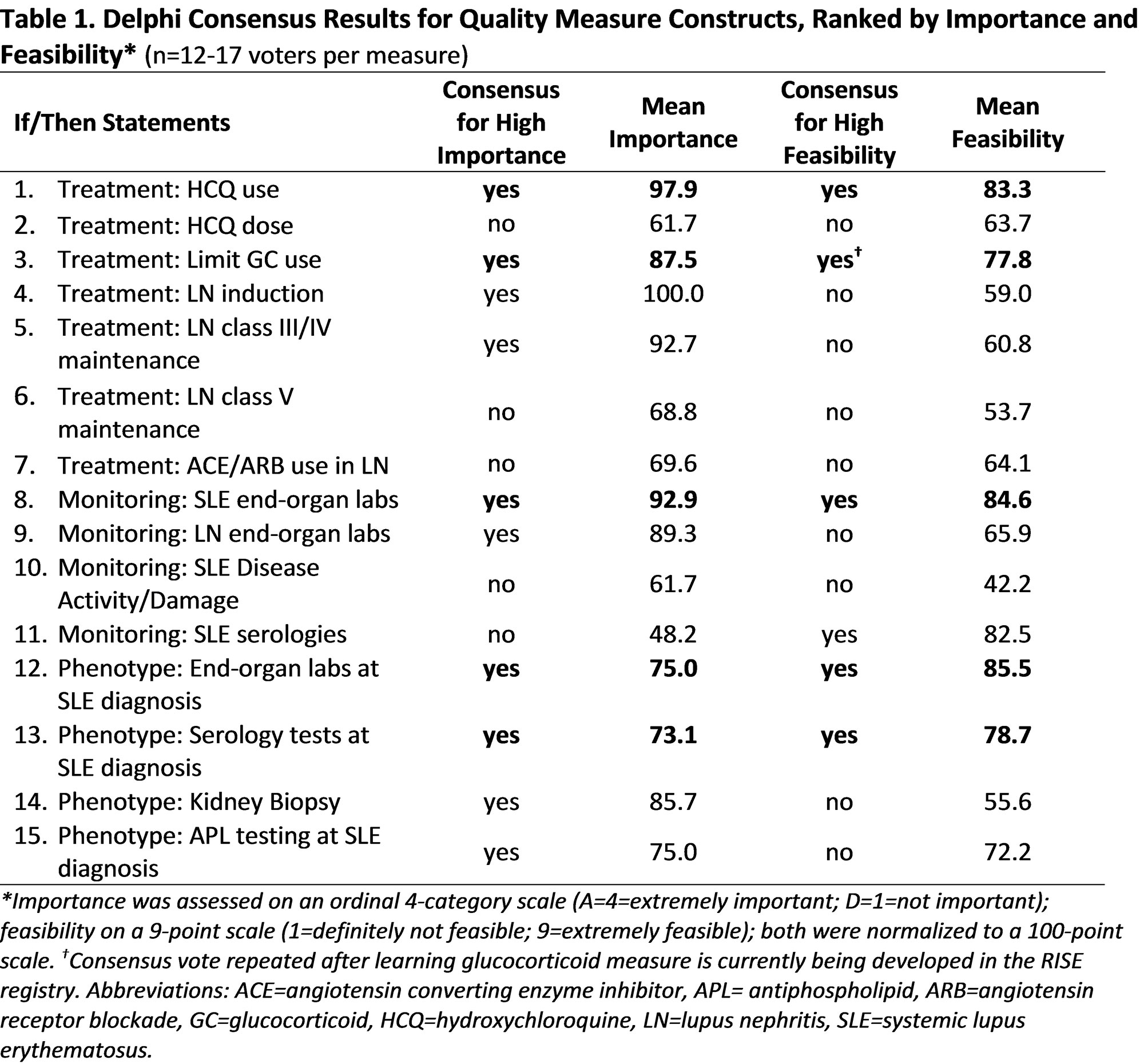
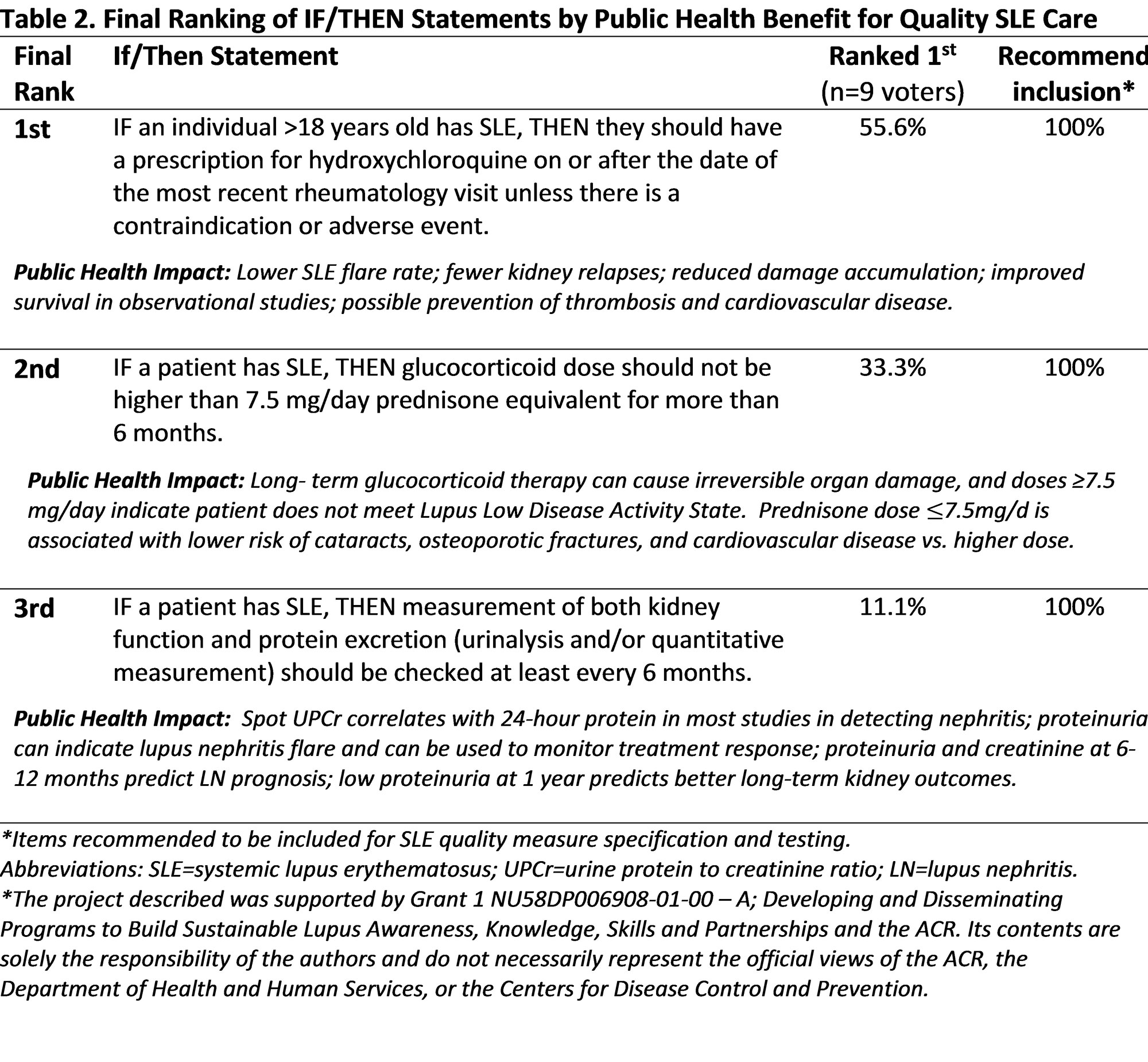
Results: Experts reviewed 10 guidelines to distill 57 quality measure constructs (18 phenotyping, 24 monitoring, 15 treatment). Fifteen highly rated constructs advanced to generate IF/THEN statements. Table 1 shows Delphi panel ratings of importance and feasibility ratings for the 15 candidate measures. Among these, 5 met high consensus for importance and feasibility: hydroxychloroquine use, limiting glucocorticoids, end-organ monitoring, serologies at diagnosis, and kidney end-organ laboratory evaluation at diagnosis. Finally, the top three recommended statements for SLE patients were: 1) hydroxychloroquine use, 2) limiting glucocorticoids (not ≥7.5mg/d for >6 months), and 3) monitoring kidney function and urine protein excretion every six months (Table 2).
Conclusion: After extensive vetting for public health impact, Delphi experts recommended three digital quality measures focused on hydroxychloroquine use, limiting glucocorticoids, and kidney monitoring. Next steps include testing candidate statements in the RISE registry for data availability and validity and working with RISE practices to optimize reliability and feasibility. Ultimately, these efforts aim to implement validated digital quality measures within US rheumatology practices to improve SLE outcomes.
To cite this abstract in AMA style:
Bartels C, Jorge A, Feldman C, Barber C, Barnado A, Bermas B, Duarte-Garcia A, Garg S, Haseley L, Jatwani S, Johansson T, Limanni A, Rodgers W, Rovin B, Santiago-Casas Y, Suter L, Ude J, Zell J, Yazdany J. Development of ACR Longitudinal Digital Quality Measures for Systemic Lupus Erythematosus: A Literature Review and Modified Delphi Consensus Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/development-of-acr-longitudinal-digital-quality-measures-for-systemic-lupus-erythematosus-a-literature-review-and-modified-delphi-consensus-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-acr-longitudinal-digital-quality-measures-for-systemic-lupus-erythematosus-a-literature-review-and-modified-delphi-consensus-study/