Session Information
Date: Wednesday, October 24, 2018
Title: 6W019 ACR Abstract: Measures of Healthcare Quality II: QI in SLE, Gout & JIA (2958–2963)
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: The U.S. Agency for Healthcare Research and Quality developed a set of general ambulatory care-sensitive conditions that may result acute care use (hospitalizations and emergency department visits), potentially preventable if high quality, timely ambulatory care was provided. We aimed to update and extend this work to develop a list of conditions specific to patients with SLE that may result in acute care use and could be potentially prevented, or their complications minimized, if timely, effective ambulatory rheumatology care had been received.
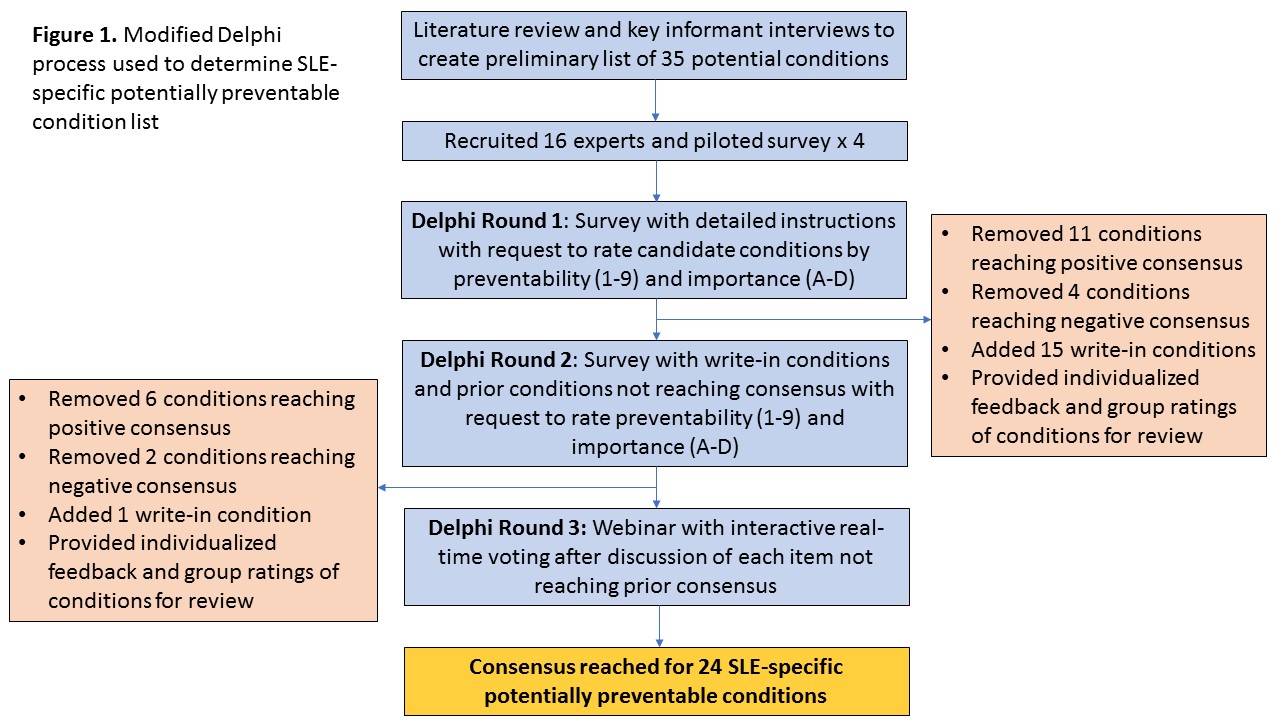
Methods: We performed a literature review and conducted key informant interviews to inform the selection of SLE-specific potentially preventable conditions. We then used a modified Delphi method to further refine the list. We assembled a panel of 16 nationally-recognized experts across the U.S. including adult (7) and pediatric (2) rheumatologists/researchers, a neurologist, an obstetrician/gynecologist, a cardiologist, two nephrologists, an infectious disease specialist and a dermatologist/rheumatologist with expertise in the SLE patient care and research. Panelists independently completed two web-based survey rounds in which they were asked to rate both the preventability (1 (preventable)-9 (not preventable)) and importance on a population scale (A (extremely important)-D (not important)) of each condition, and to provide comments and additional conditions for consideration. Analysis was guided by the RAND-UCLA Appropriateness Method. Consensus was determined by median scores ≤3, <3 extreme responses in the contrary direction, and ≥65% with high [A or B] importance ratings. A final webinar was used to adjudicate conditions that did not reach prior consensus.
Results: Thirty-five potential conditions were initially considered (Figure 1). The response rate was 100% for both survey rounds and 14/16 panelists participated in the webinar process. 11 conditions met consensus criteria from round one, 6 from round two and 7 from the final round. Included conditions fell into 3 broad categories: medication-related toxicities (12 conditions), vaccine-preventable illnesses (5), or SLE-related complications (7) (Table).
Conclusion: SLE-specific potentially preventable adverse conditions include a diverse set of infectious, medication-related and SLE disease-related complications. This set of conditions will allow for identification of high risk SLE patients and facilitate targeted interventions with the goal of reducing preventable acute care use and adverse outcomes.
|
Table. Final set of SLE-specific potentially preventable conditions meeting consensus criteria as potentially preventable and important on a population level among patients with known SLE+ |
|||||
|
SLE-Specific Potentially Preventable Conditions |
Preventability Rating 1 (definitely preventable) – 9 (not preventable) |
Importance Rating*N (%) A (very important) – D (not important on a population level) |
|||
|
|
Median (range) |
A |
B |
C |
D |
|
Medication-related toxicities/complications |
|||||
|
Vision loss from hydroxychloroquine toxicity (N=15) |
1 (1-3) |
13 (87) |
1 (7) |
— |
1 (7) |
|
Teratogenesis while on teratogenic medications (N=16) |
1.5 (1-8) |
16 (100) |
— |
— |
— |
|
Opioid overdose (N=13) |
2 (1-4) |
9 (69) |
4 (31) |
— |
— |
|
Pneumocystis pneumonia while on moderate/high dose corticosteroids (N=15) |
2 (1-4) |
8 (53) |
4 (27) |
2 (13) |
1 (7) |
|
Chronic opioid use (N=13) |
2 (1-4) |
7 (54) |
6 (46) |
— |
— |
|
Vascular thrombosis in the setting of estrogen-based contraception and +APLAs (N=14) |
2.5 (1-4) |
9 (64) |
5 (36) |
— |
— |
|
Gastrointestinal bleed while on corticosteroids, NSAIDs or anticoagulation (N=12) |
2.5 (2-4) |
3 (25) |
9 (75) |
— |
— |
|
Uncontrolled steroid-induced diabetes (N=15) |
3 (1-6) |
10 (67) |
4 (27) |
1 (7) |
— |
|
Osteoporotic fracture while on corticosteroids (N=15) |
3 (2-6) |
9 (60) |
6 (40) |
— |
— |
|
Premature ovarian failure/infertility following standard dose cyclophosphamide without ovarian preservation therapy (N=13) |
3 (1-5) |
8 (62) |
5 (38) |
— |
— |
|
Spontaneous abortion while on teratogenic medications (N=15)# |
3 (1-9) |
8 (53) |
5 (33) |
1 (7) |
— |
|
Avascular necrosis while on prolonged corticosteroids (N=10) |
3 (3-6) |
3 (30) |
6 (60) |
1 (10) |
— |
|
Vaccine-preventable illnesses |
|
|
|
|
|
|
High-grade cervical dysplasia/cervical cancer (N=14) |
2 (1-8) |
12 (86) |
2 (14) |
— |
— |
|
Influenza (N=15) |
2 (2-3) |
9 (60) |
5 (33) |
1 (7) |
— |
|
Herpes zoster (N=11) |
2 (2-5) |
6 (55) |
5 (45) |
— |
— |
|
Bacterial meningitis (N=11) |
2 (2-6) |
4 (36) |
4 (36) |
3 (27) |
— |
|
Pneumococcal pneumonia (N=14) |
3 (2-5) |
9 (64) |
4 (29) |
1 (7) |
— |
|
SLE-related complications |
|||||
|
Vascular thrombosis in patients with APS (N=15) |
2 (1-4) |
12 (80) |
3 (20) |
— |
— |
|
Embolic stroke in patients with APS (N=15) |
2.5 (2-5) |
10 (67) |
5 (33) |
— |
— |
|
Lupus flare in the absence of UV protection (N=12) |
2.5 (1-6) |
5 (42) |
7 (58) |
— |
— |
|
Acute renal failure among patients with lupus nephritis (N=15) |
3 (2-8) |
12 (75) |
3 (25) |
— |
— |
|
Recurrent myocardial infarction (N=11) |
3 (2-7) |
9 (82) |
2 (18) |
— |
— |
|
Obstetrical complications in patients with APS (N=12) |
3 (1-4) |
8 (67) |
4 (33) |
— |
— |
|
Neonatal lupus/congenital heart block with a mother with +anti-Ro or anti-La antibodies (N=11) |
3 (2-5) |
7 (64) |
4 (36) |
— |
— |
|
+Panelists with specific expertise could opt-out of rating conditions outside of their area; results presented for the final round for which consensus for inclusion was reached *Importance was defined on the population level, including consideration of the prevalence of the outcome in the SLE population #One response of “not applicable” for the importance rating APLAs= antiphospholipid antibodies, APS=antiphospholipid syndrome |
|||||
To cite this abstract in AMA style:
Feldman CH, Speyer C, Ashby R, Bermas BL, Bhattacharyya S, Chakravarty E, Everett B, Ferucci E, Hersh AO, Marty F, Merola JF, Ramsey-Goldman R, Rovin B, Son MB, Tarter L, Waikar S, Yazdany J, Weissman J, Costenbader K. Development of a Set of Potentially Preventable Adverse Conditions Specific to Lupus: A Delphi Consensus Study [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/development-of-a-set-of-potentially-preventable-adverse-conditions-specific-to-lupus-a-delphi-consensus-study/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-set-of-potentially-preventable-adverse-conditions-specific-to-lupus-a-delphi-consensus-study/