Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Elevated and coordinated expression of multiple Interferon-regulated genes (IRGs) in peripheral blood cells is present in most systemic lupus erythematosus (SLE) patients. Here we describe the development of a validated quantitative PCR (qPCR) test to accurately measure relative levels of IRGs in SLE blood at baseline and over the course of clinical trials.
Methods: A cross sectional SLE cohort (n=150) and a healthy cohort (n=70) were used for qPCR development. Subject samples from two clinical trials with rontalizumab (anti-IFN alpha), Phase 1 with mild to moderate lupus (n=60) and Phase 2 with moderate to severe lupus (ROSE, n=238) were used for validation. Expression of type I IRGs was determined by expression microarray and qPCR analysis of peripheral blood RNA. Relationships between IRGs were assessed using Spearman’s rank correlation. Exploratory hypothesis testing between patient subsets was performed using Wilcoxon rank sum or Fisher’s exact tests.
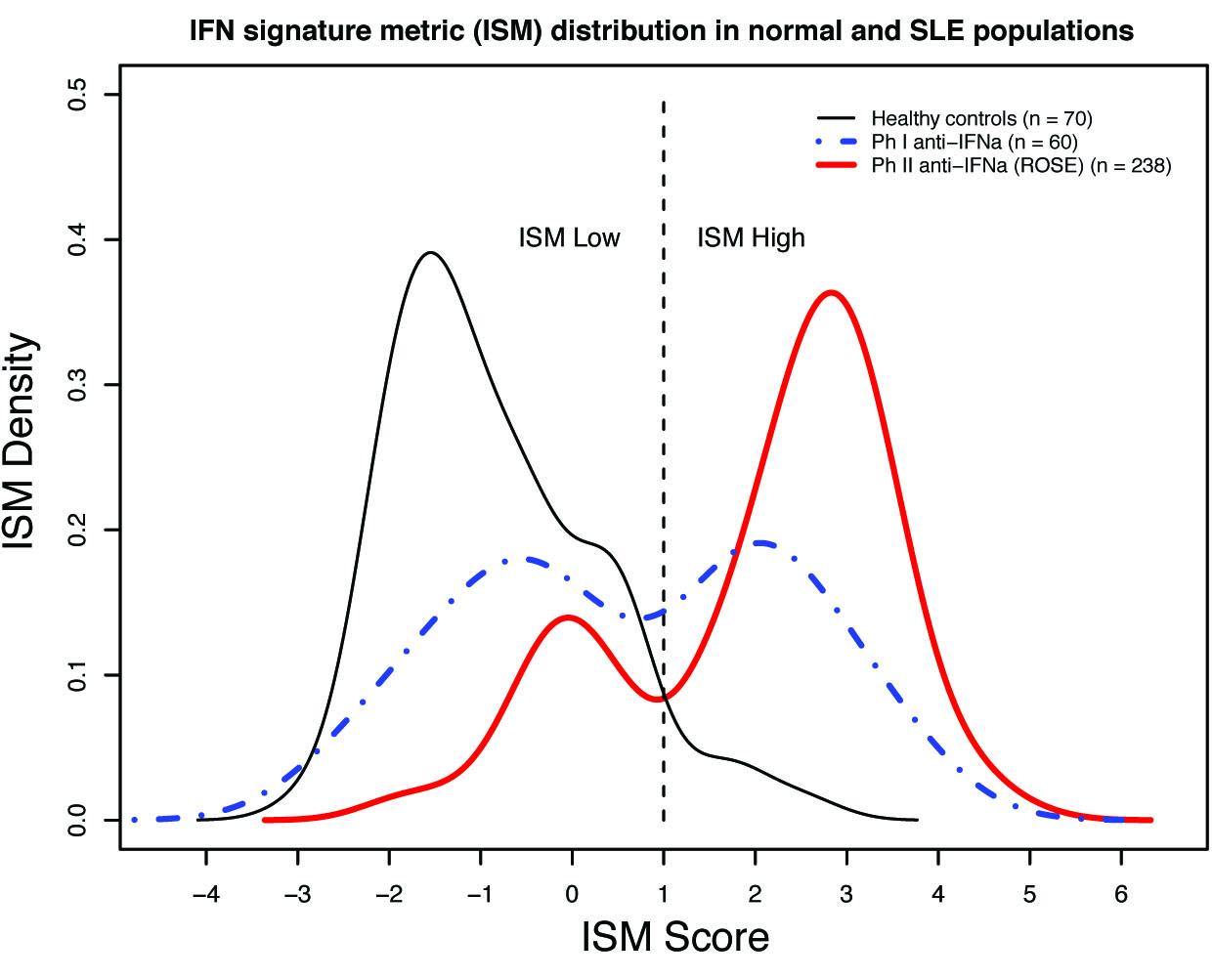
Results: Unsupervised hierarchical clustering revealed a region comprised of a high density of IRGs and a representative 3-gene combination (r >0.98) was chosen for measurement by qPCR. An Interferon Signature Metric (ISM) score was derived from the average normalized expression of these 3 genes. ISM scores in the Rontalizumab trials followed a bimodal distribution compared with controls. Determination of threshold cutoff for ISM high vs. low designation was based upon the 95th percentile of the ISM scores of healthy controls, allowing discrimination of the two modes of distribution in SLE. In the ROSE trial there were no differences in disease activity as measured by BILAG and SELENA-SLEDAI between identified ISM high and ISM low subjects. Further, longitudinal assessment of subjects in the placebo arm of the ROSE trial followed over 36 weeks showed that the probability of maintaining either ISM low or high status was 94% (95% CI 91%-97%).
Conclusion: The ISM test accurately quantifies the bimodal blood Interferon Signature in SLE subjects. ISM high status was not associated with increased BILAG and SELENA-SLEDAI-defined disease activity. ISM status was observed to be stable over 36 weeks of longitudinal assessment. The ISM test may provide useful information in clinical trials of targeted therapies and in the understanding of disease heterogeneity in SLE.
Disclosure:
B. C. Richardson,
None;
W. P. Kennedy,
Genentech, Inc,
3,
Roche Pharmaceuticals,
1,
Merck co,
1;
J. C. Davis Jr.,
Roche Pharmaceuticals,
1,
Genentech, Inc.,
3;
R. Maciuca,
Roche Pharmaceuticals,
1,
Genentech , Inc.,
3;
A. Morimoto,
Roche Pharmaceuticals,
1,
Genentech, Inc.,
3;
J. M. McBride,
Roche Pharmaceuticals,
1,
Genentech, Inc,
3;
A. R. Abbas,
Genentech, Inc,
3,
Roche Pharmaceuticals,
1;
T. W. Behrens,
Roche Pharmaceuticals,
1,
Genentech, Inc.,
3;
M. J. Townsend,
Roche Pharmaceuticals,
1,
Genentech, Inc.,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-quantitative-pcr-method-to-determine-interferon-signature-metric-status-in-sle-patients-distribution-and-clinical-serological-associations-in-two-lupus-clinical-trials/