Session Information
Date: Tuesday, November 7, 2017
Title: Systemic Lupus Erythematosus – Human Etiology and Pathogenesis Poster II
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: There is a critical need to define the cells that mediate tissue damage in lupus nephritis. Here we aimed to establish a protocol to preserve lupus nephritis kidney biopsies and urine cell samples obtained at multiple clinical sites for subsequent isolation and transcriptomic analysis of single cells.
Methods: We developed a method to cryopreserve intact, viable kidney tissue in a 10% DMSO-containing solution. Viable cryopreserved kidney tissue from tumor nephrectomies and lupus nephritis kidney biopsies were disaggregated by enzymatic digestion and compared to freshly processed tissues. Cell yields and cell composition were assessed by flow cytometry. Transcriptomes of flow-sorted CD45+ leukocytes and CD10+ kidney epithelial cells were evaluated by low-input and single cell RNA-seq.
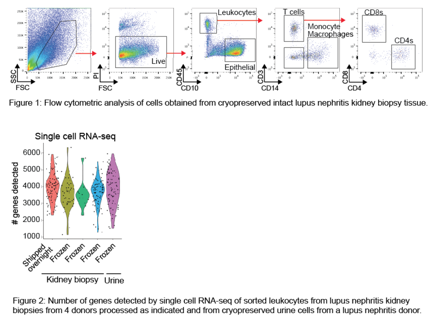
Results: Cryopreserved kidney tissue from tumor nephrectomies and lupus nephritis biopsies can be thawed and dissociated to yield intact, viable leukocytes and epithelial cells. Cryopreservation of intact kidney tissue provides higher epithelial cell yields compared to cryopreservation of single cell suspensions from dissociated kidneys. Epithelial cell and leukocyte lineage markers are easily detected on cells from cryopreserved kidney tissue by flow cytometry (Figure 1). Cell yields and flow cytometric cell phenotypes are comparable between cryopreserved kidney samples and paired kidney samples shipped overnight on wet ice. High-quality single cell and low-input transcriptomic data were generated from flow-sorted leukocytes from both cryopreserved lupus nephritis kidney biopsies and urine, typically with 3000-4000 genes detected in single cell transcriptomes (Figure 2).
Conclusion: We propose that this method of acquisition of viable cells from cryopreserved intact kidney tissue may serve as a model for robust, centralized cellular analyses of kidney samples acquired in multi-site clinical studies. More broadly, this strategy enables accumulation of a valuable biobank of tissues containing viable cells that can be used for multiple downstream analyses.
To cite this abstract in AMA style:
Rao D, Berthier CC, Arazi A, Davidson A, Liu Y, Browne E, Eisenhaure T, Chicoine A, Lieb D, Smilek D, Tosta P, Lederer J, Brenner M, Hildeman D, Woodle ES, Wofsy D, Anolik JH, Kretzler M, Hacohen N, Diamond B. Development of a Protocol for Single Cell Transcriptomics of Cells from Cryopreserved Lupus Nephritis Kidney Tissue and Urine for the Accelerating Medicines Partnership RA/SLE Network [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/development-of-a-protocol-for-single-cell-transcriptomics-of-cells-from-cryopreserved-lupus-nephritis-kidney-tissue-and-urine-for-the-accelerating-medicines-partnership-rasle-network/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-protocol-for-single-cell-transcriptomics-of-cells-from-cryopreserved-lupus-nephritis-kidney-tissue-and-urine-for-the-accelerating-medicines-partnership-rasle-network/