Session Information
Date: Monday, November 8, 2021
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: RA patients who respond inadequately to first-line DMARDs usually progress to a biologic DMARD. Treatment response to both DMARDs and biologics is heterogeneous within RA patients. Choosing the right treatment early is vital, but prediction models from the literature are largely based on complex biomarkers such as genetics. Here, we develop a prediction model, using only clinical baseline covariates, to predict 3-month treatment response to the commonly used anti-TNF-α drug Etanercept (ETN).
Methods: Using data from the Biologics in RA Genetics and Genomics Study Syndicate (BRAGGSS), we identified patients treated with ETN or biosimilars. Patients were included for analysis if they had complete data to calculate a Disease Activity Score of 28 Joints (DAS28) at baseline and 3 months. DAS28 was then used to calculate EULAR response groups. We retained patients with complete baseline data as poor-responders if they stopped treatment early due to inefficacy. We created two datasets, containing all samples with complete CRP (N = 778), and ESR data (N = 693), respectively. Samples with complete CRP and ESR data, appear in both datasets. Multivariable logistic regression models were fitted using baseline clinical covariates, to differentiate poor (y = 1) from good and moderate (y = 0) responders at 3 months. Table 1 shows the baseline statistics of the covariates included in the models, for both created datasets. Multiple imputation by chained equations was used to impute missing data, and models were internally validated via bootstrapping. We report model discrimination as measured by the area under the ROC curve (AUC), and model calibration via the calibration slope. Model parameters are described using β coefficients and odds-ratios (ORs).
Results: Both models are well calibrated with moderate discriminatory ability. Adjusted for optimism, the CRP model achieves an AUC of 0.658 (IQR 0.655-0.66), with a calibration slope of 0.767 (IQR 0.753-0.781). The ESR model shows a slightly higher AUC of 0.671 (IQR 0.67-0.672), with a marginally worse calibration slope of 0.759 (IQR 0.751-0.767). The calibration curve (figure 1) shows predictions are accurate, but that the models rarely predict high probabilities of poor response, which helps explain the moderate AUCs. Model parameters are mostly similar across the two models, and show HAQ to have the biggest impact, with β coefficients of 0.893 (OR 2.44) and 0.8528 (OR 2.35). Additionally, if this is their first biologic treatment, a patient’s odds of having a poor response are significantly reduced (OR 0.45 and 0.48 for CRP and ESR, respectively).
Conclusion: Though competitive with existing genetic models from the literature, clinical features alone have insufficient discriminatory ability to predict response to ETN. These models serve as a baseline to investigate and evaluate the use of more complex models incorporating biomarkers to further improve prediction of treatment outcomes in RA. All model parameters are provided (see table 2) to facilitate external validation as well as the further development required to create models that can eventually inform treatment decisions.
 Table 1: Baseline statistics for the covariates used in the different models. % in brackets indicate the percentage of missing data for this covariate
Table 1: Baseline statistics for the covariates used in the different models. % in brackets indicate the percentage of missing data for this covariate
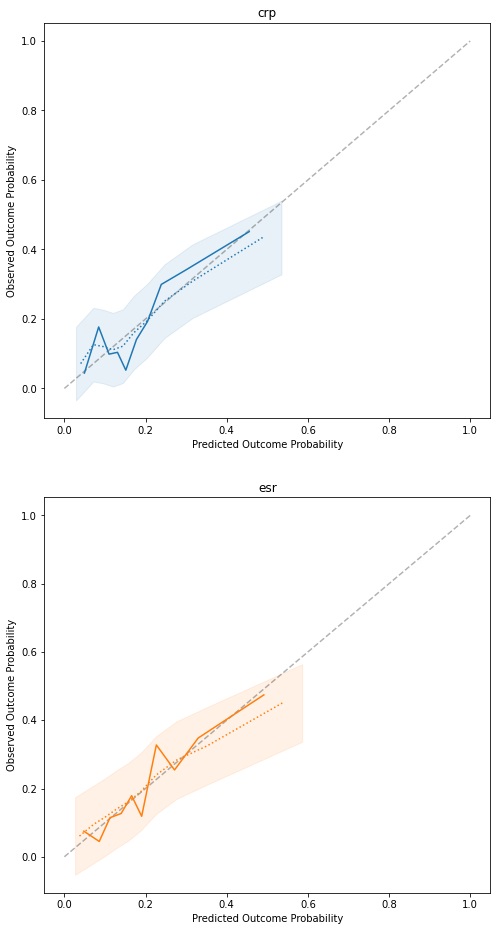 Figure 1: Calibration curves for the fitted models. The dotted line and shaded area represent the average bootstrap calibration curve and its standard deviation.
Figure 1: Calibration curves for the fitted models. The dotted line and shaded area represent the average bootstrap calibration curve and its standard deviation.
 Table 2: Model parameters and their respective odds ratio (OR)
Table 2: Model parameters and their respective odds ratio (OR)
To cite this abstract in AMA style:
Stadler M, Ling S, Nair N, Isaacs J, Hyrich K, Morgan A, Wilson A, Plant D, Barton A, Bowes J. Development of a Multivariable Prediction Model for Treatment Response to Etanercept in a Multi-centre Cohort of Patients with Established RA [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/development-of-a-multivariable-prediction-model-for-treatment-response-to-etanercept-in-a-multi-centre-cohort-of-patients-with-established-ra/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-multivariable-prediction-model-for-treatment-response-to-etanercept-in-a-multi-centre-cohort-of-patients-with-established-ra/
