Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Chronic nonbacterial osteomyelitis (CNO) is an inflammatory bone disease that can result in bone deformity, persistent pain and pathological fractures which is frequently treated empirically. Clinical trials to compare and evaluate medication efficacy necessitates quantifying a minimal clinically important difference (MCID). We aimed to develop and validate this metric in CNO.
Methods: A Delphi survey on priorities for defining MCID was sent to providers via the Childhood Arthritis and Rheumatology Research Alliance (CARRA’s) CNO Workgroup (180 members). Using the survey’s results, 664 cases were identified from over 3000 real world patient visits from our multisite prospective registry (CHronic nonbacterial Osteomyelitis International Registry, CHOIR) to define MCID. The interval between visits was between 3 and 12 months. At least 1 or more values at the follow up visits had improved while no more than 1 value worsened. An expert panel of 13 international pediatric rheumatologists and 7 patient/caregivers reviewed the cases individually to determine if they showed MCID. Cases which did not reach consensus (80% agreement) were evaluated by >5 reviewers at an in-person consensus meeting. Reviewers were asked to vote whether a case demonstrated MCID. A decision tree was used to determine the cutoff point that best differentiated patients with and without MCID, and the model was validated using 10-fold cross-validation to ensure generalizability.
Results: A total of 80 providers (44% response rate) from 5 continents completed the survey. The frequency of items selected were listed and the absolute and relative change were reported (Figure 1). Cohort characteristics and parameters extracted from paired baseline and follow up visits are in Table 1. 638 (96%) of 664 paired visits reached consensus. 423 were classified as MCID, 215 as no MCID and 26 were inconclusive. A generalized linear model analysis showed that the change of pain level (p=0.03), baseline MRI lesion count (p=0.02), change of MRI lesion count (p< 0.001) and change of active spinal lesion on MRI (p< 0.02) were associated with the classification of MCID. The median [IQR] change of clinical disease activity score (CDAS) was -7 [-11,-4] in cases with MCID, compared to -2 [-5, 0] in those without MCID (p< 0.001). Decision trees based on all parameters or CDAS alone with accuracy of 0.73 and 0.71, respectively are in Figure 2.
Conclusion: A Delphi survey identified important variables to include when defining MCID per providers. We developed definitions for MCID, and specified measurement characteristics. A 30% improvement in these variables with improvement of CDAS by 3 were considered meaningful by providers and consensus data.
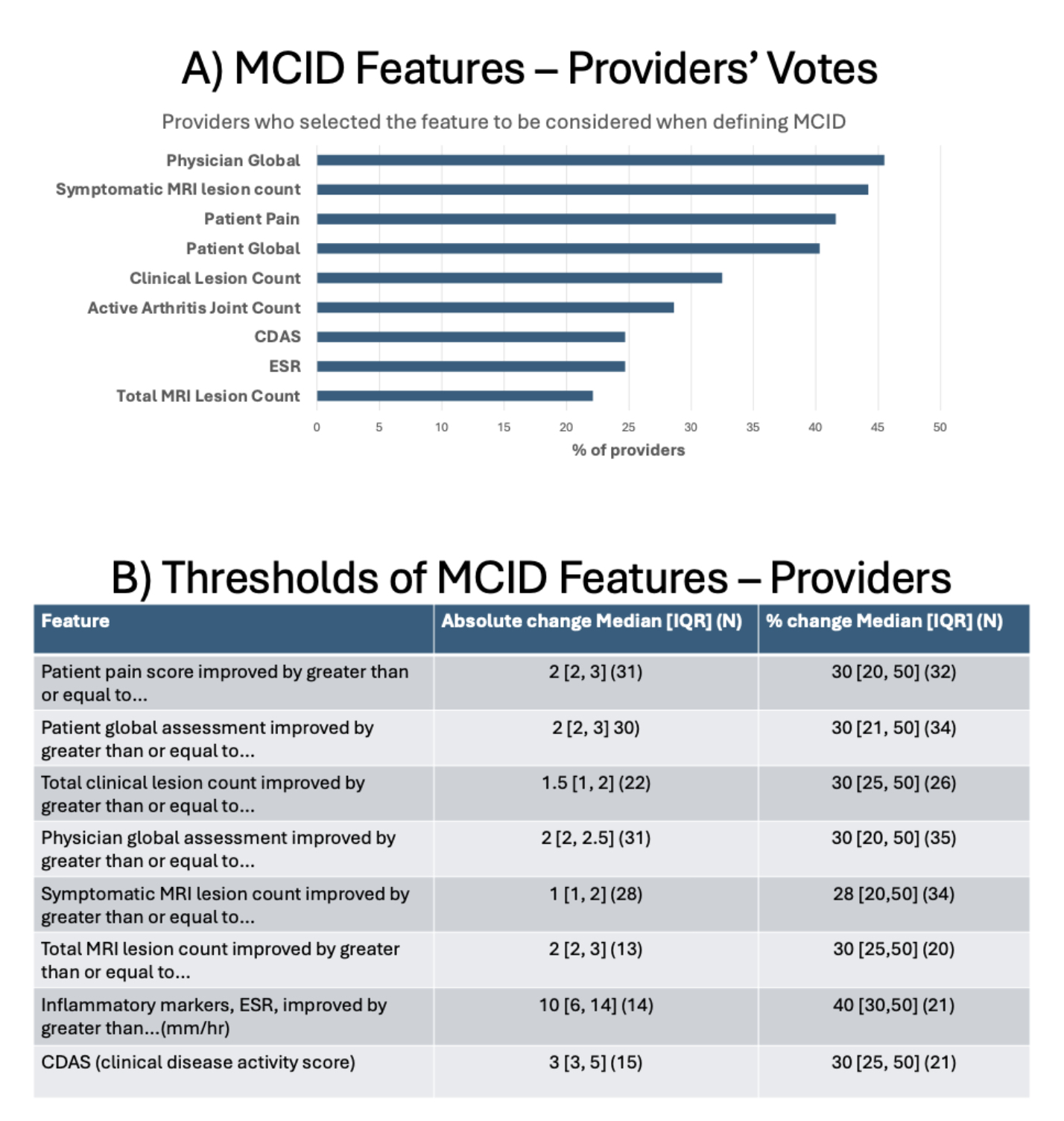 Figure 1: Provider Priorities in Defining Minimal Clinically Important Difference in CNO Delphi Survey
Figure 1: Provider Priorities in Defining Minimal Clinically Important Difference in CNO Delphi Survey
Results: Frequencies and Thresholds
.jpg) Table 1: Characteristics of Cohorts Used to Develop Definitions of Minimal Clinically Important Difference in CNO
Table 1: Characteristics of Cohorts Used to Develop Definitions of Minimal Clinically Important Difference in CNO
.jpg) Figure 2: Decision Pathways for Defining Minimal Clinically Important Difference in CNO
Figure 2: Decision Pathways for Defining Minimal Clinically Important Difference in CNO
To cite this abstract in AMA style:
Nuruzzaman F, Rosenwasser N, Wang X, Klein A, Muse I, Balay-Dustrude E, Nguyen M, Deng E, Akikusa J, Basiaga M, Bergstrom L, Dedeoglu F, Huang B, King J, Lapidus S, Lee T, Levesque C, Lenert A, Lim L, Martin K, Murray E, Oliver M, Onel K, Özen S, Potts L, Stern S, Villaverda R, Wu E, laxer R, Ferguson P, Lovell D, Zhao Y. Development and Validation of Minimal Clinically Important Difference for Children with Chronic Nonbacterial Osteomyelitis Using a Consensus and Data-Driven Approach [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/development-and-validation-of-minimal-clinically-important-difference-for-children-with-chronic-nonbacterial-osteomyelitis-using-a-consensus-and-data-driven-approach/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-validation-of-minimal-clinically-important-difference-for-children-with-chronic-nonbacterial-osteomyelitis-using-a-consensus-and-data-driven-approach/
