Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Chronic nonbacterial osteomyelitis (CNO) is an autoinflammatory bone disease. The diagnosis of CNO is made by recognizing typical clinical and imaging presentation and excluding mimicking diseases. In patients with clinical characteristics concerning for mimicking diseases or those with atypical presentation of CNO, a bone biopsy may be necessary to aid the clinical diagnosis. We aimed to develop and validate a novel score system to identify subsets of patients with very low or very high probability of CNO, prior to the decision of whether to do a bone biopsy.
Methods: A total of 960 cases collected from 31 hospital centers across 6 continents were used. Cohort was comprised of 672 pediatric CNO cases and 288 CNO mimicker cases. Demographic, clinical, laboratory, and imaging findings were collected. At least 12 months follow up was required for CNO cases and for mimicker cases unless confirmatory diagnostic and laboratory findings of a mimic condition were uncovered. Cases were randomly assigned into development and validation cohorts and the development cohort was used for model creation. Imaging findings plus routinely measured features were used in logistic regression with stepwise deletion. Regression coefficients from this model were rounded to integer weights to compute a score, with higher scores indicating a greater likelihood of CNO. This model was used to sort patients into ordered categories based on likelihood of CNO, then applied to the Validation Cohort to obtain an unbiased estimate of model performance.
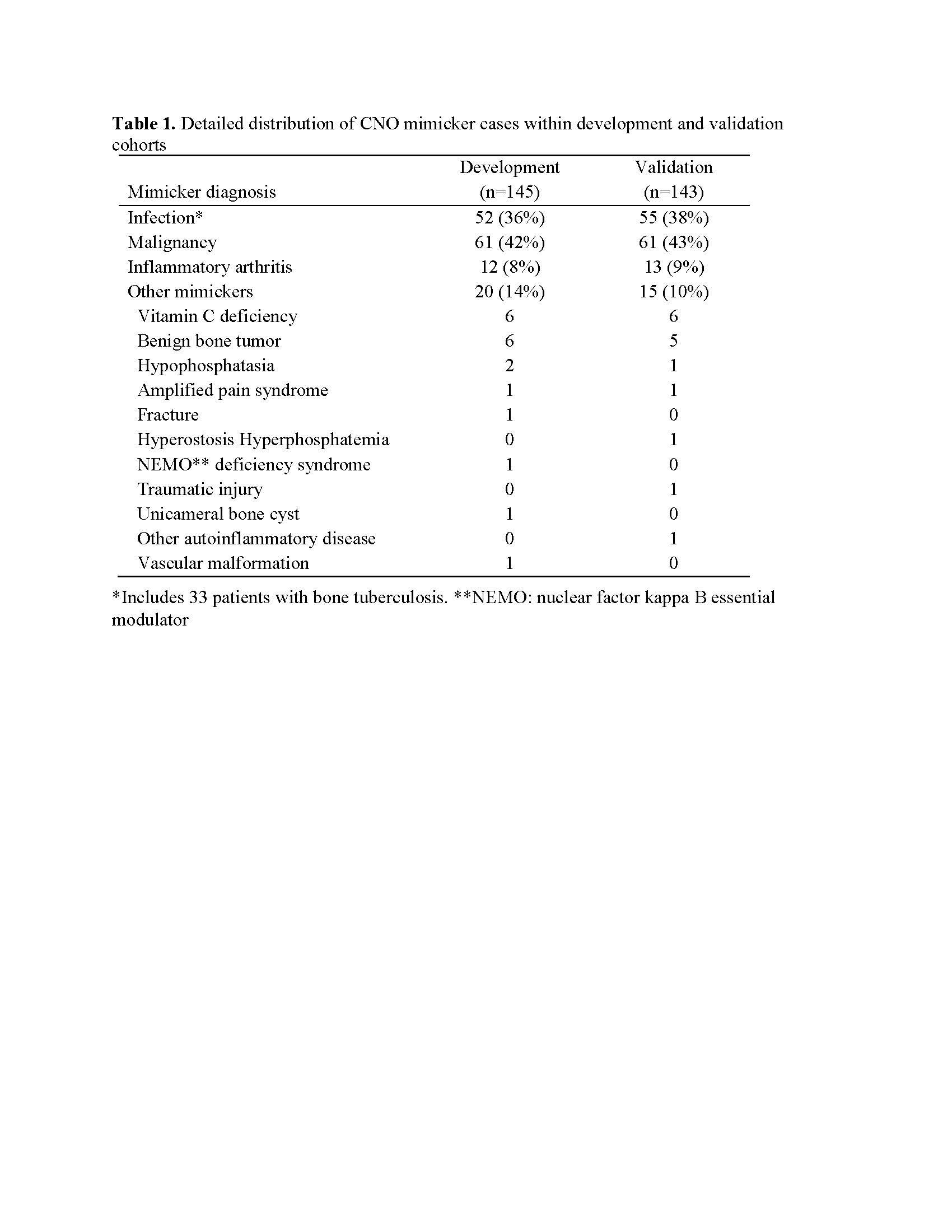
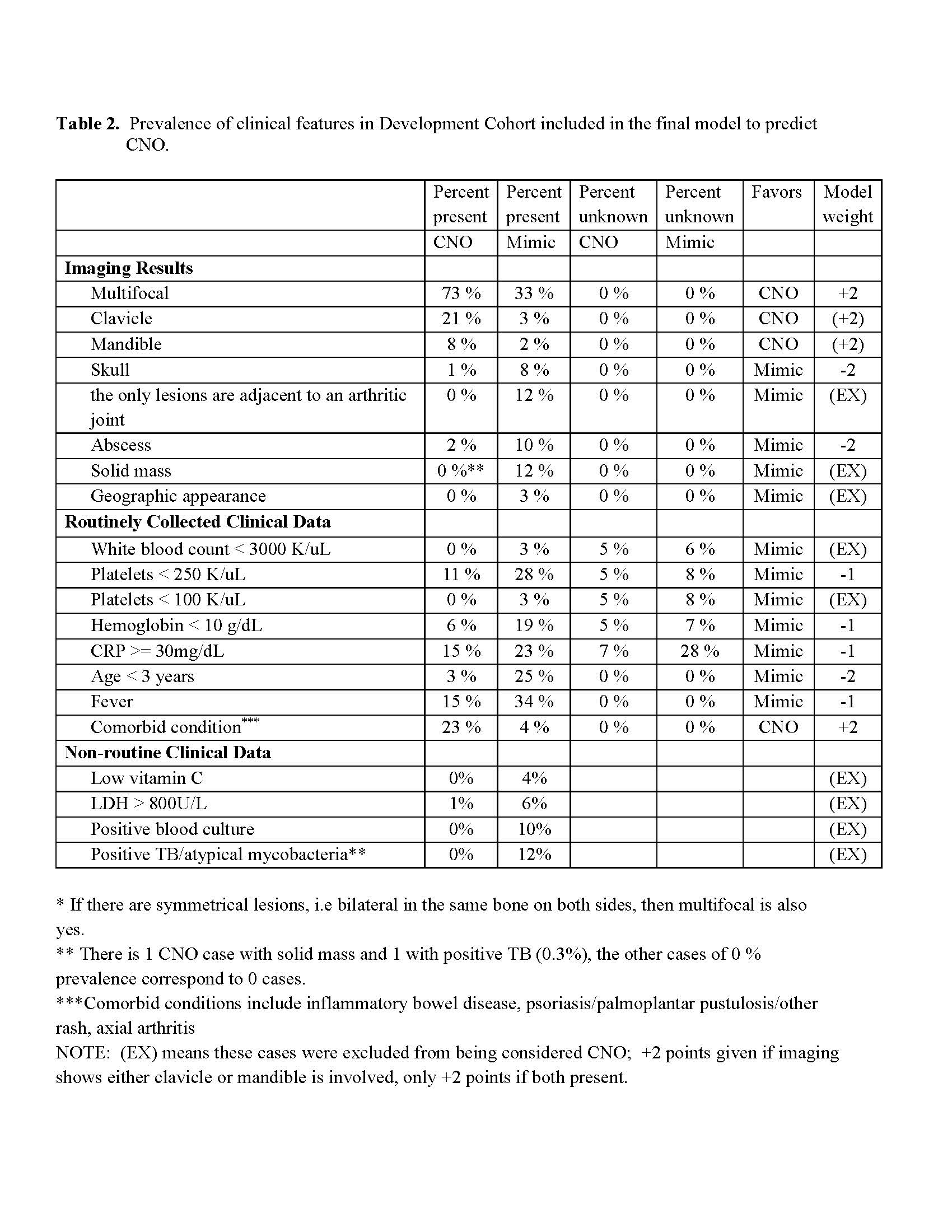
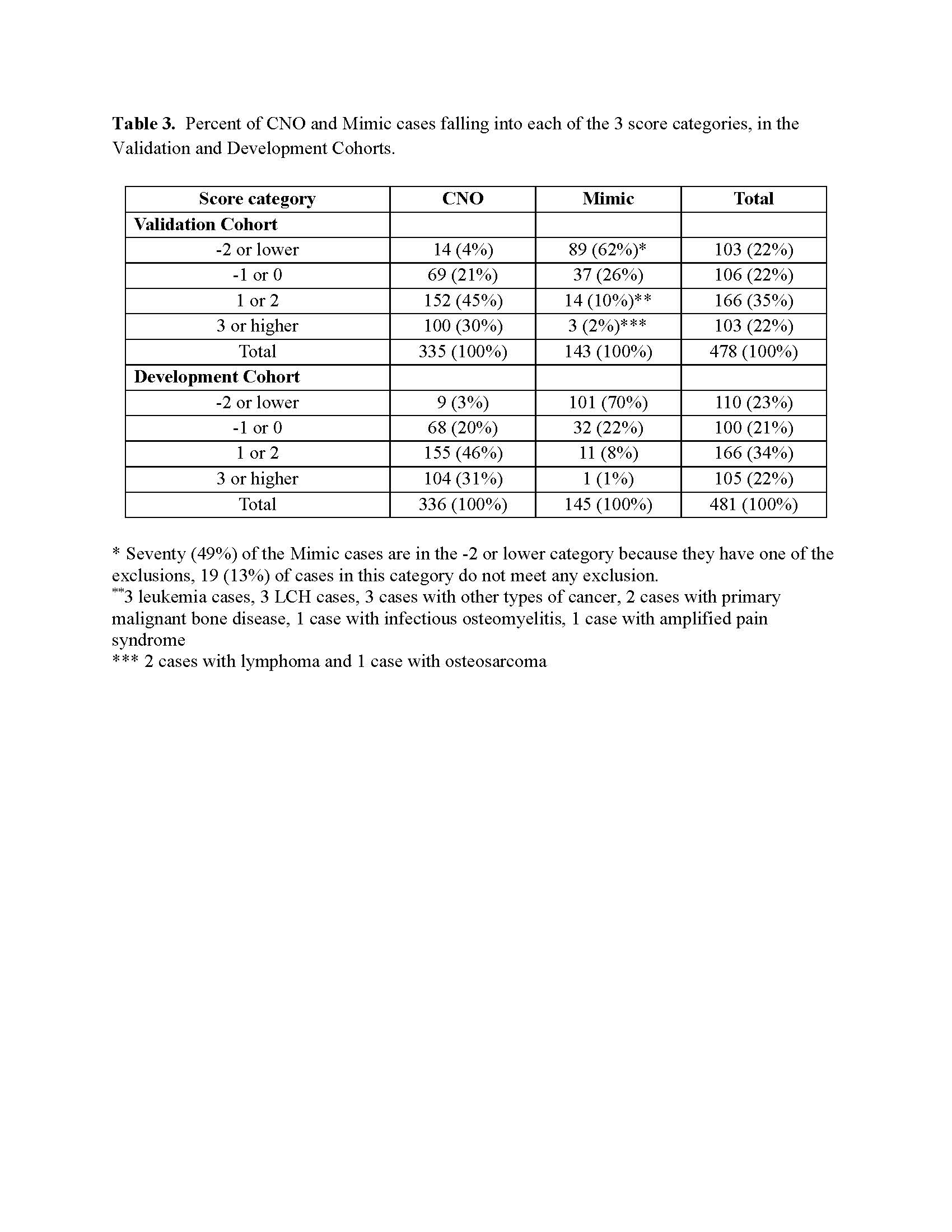
Results: About 80% of mimicker cases in the total sample were from infectious or malignant causes which often require bone biopsies (Table 1). Imaging features and routinely collected features considered for inclusion in the initial model are shown in Table 2. Clinical characteristics considered for the initial model are shown in Table 1 along with the corresponding weights for the final model. Table 3 shows results of the final model applied to the Validation and Development Cohorts, with scores collapsed into 4 categories. If a threshold of ≥ 1 is used, sensitivity is 75% and specificity is 88%. We propose: 1) for cases score ≤ -2, CNO is unlikely and hence focus should be on determining which mimic condition is present, with bone biopsy only if useful for that purpose ; 2) for cases score -1 or 0, bone biopsy is likely to be useful to determine whether infection or malignancy is present; 3) for cases score ≥ 1, CNO is highly likely and a bone biopsy may required if imaging or clinical features raise the suspicion of malignancy or infection. Among the CNO cases scored ≥ 1 (n=252) in the Validation Cohort, 142 (56%) had a bone biopsy. Under our proposal, many of these biopsies could be avoided.
Conclusion: Our novel CNO score system derived from an international cohort showed it is possible to separate patients into categories based on minimally invasive tests. This information can be useful in identifying cases for which a bone biopsy would be appropriate.
To cite this abstract in AMA style:
Pooni R, Menashe S, Oliver M, Schnabel A, Wu E, Wang Z, Yang C, Marino A, Aguiar C, Akikusa J, Akca U, Almeida B, Appenzeller S, Basaran O, Basiaga M, Cabral D, Capponi M, Donaldson n, Egeli B, Fox E, insalaco A, Iyer R, Jansson A, Kostik I, Kostik M, Kovalick L, Kozu K, Lapidus S, Lee T, Lenert A, Mahmood K, Marrani E, Mosa D, Mushkin A, Nuruzzaman F, Onel K, Pardeo M, Pham T, Potts L, Ramanan A, Ravelli A, Rogers N, Muse I, Romano M, Roseenwasser n, Sato T, Simonini G, Soep j, Stern S, Theos A, Tucker L, Vogel L, Yasin S, Bouchalova K, Hendry A, Cain K, Girschick H, Dedeoglu F, Hedrich C, Laxer R, Ferguson P, Ozen S, Zhao Y. Development and Validation of a Novel Score System to Guide Diagnostic Procedures in Children with Concerns of Chronic Nonbacterial Osteomyelitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/development-and-validation-of-a-novel-score-system-to-guide-diagnostic-procedures-in-children-with-concerns-of-chronic-nonbacterial-osteomyelitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-validation-of-a-novel-score-system-to-guide-diagnostic-procedures-in-children-with-concerns-of-chronic-nonbacterial-osteomyelitis/