Session Information
Date: Tuesday, November 19, 2024
Title: Abstracts: Miscellaneous Rheumatic & Inflammatory Diseases II
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: VEXAS (Vacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic) syndrome is a recently identified autoinflammatory disorder with a heterogenous presentation. Patients may experience delays in diagnosis in favor of better known autoimmune or hematologic conditions. Studies focused on identifying which patients should be considered for confirmatory UBA1 testing are limited. Thus, we aimed to design predictive models to efficiently inform clinicians about when and who to test for VEXAS syndrome.
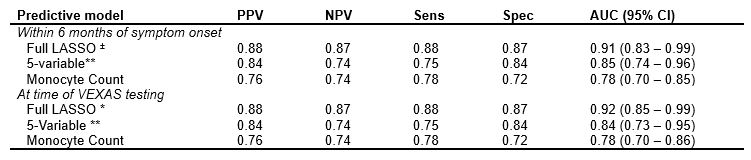
Methods: Patients with inflammatory/hematologic abnormalities who had UBA1 testing for suspected VEXAS syndrome at our institution were identified. An extensive list of clinical and laboratory features related to VEXAS syndrome was developed and abstracted from the medical records. Features were examined at two time points: within 6 months of onset of symptoms suspected to be due to VEXAS and at the time of VEXAS testing. Two multivariable models were derived via the least absolute shrinkage and selection operator (LASSO) method, with nested cross-validation used to estimate performance on unseen data. A “full” LASSO model utilized all features selected by the LASSO method, and a second, 5-variable LASSO model utilized only the top 5 most discriminating features from the full model. A third, single variable model, utilizing absolute monocyte count, was also examined. Area under the receiver operating characteristic curve (AUC) analysis was used to assess each model’s ability to discriminate between positive and negative cases (presence or absence of UBA1 mutation on genetic testing respectively). Additional descriptive assessment of each model, including sensitivity, specificity, and positive and negative predictive values were estimated by maximizing Youden’s index.
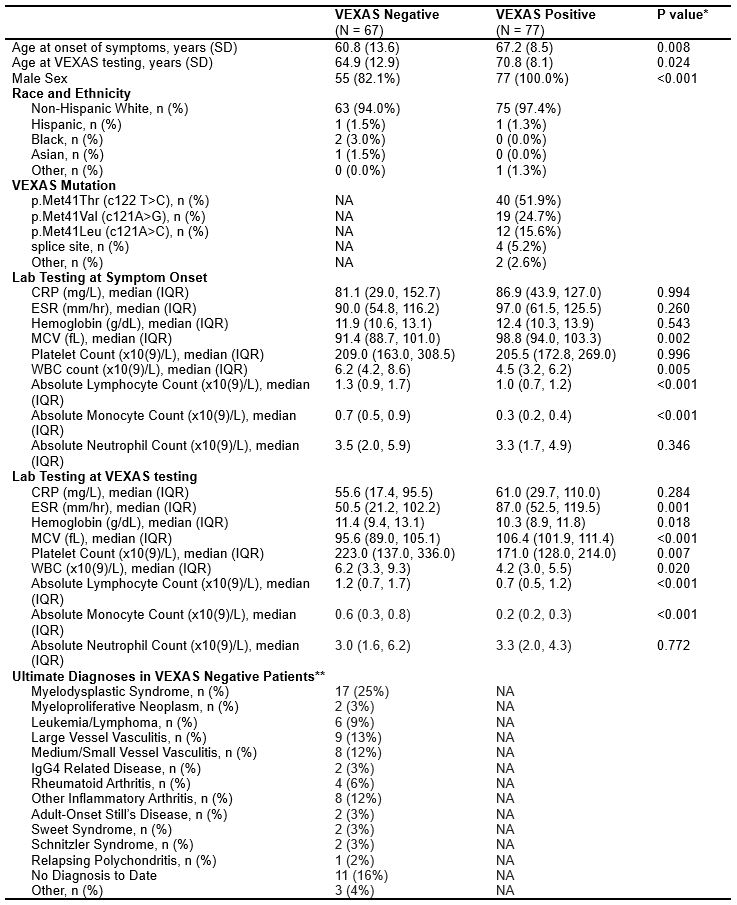
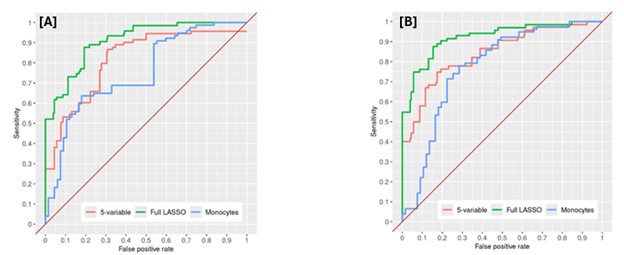
Results: A total of 144 patients underwent UBA1 genetic testing for VEXAS, of which 77 patients (all men, 97.4% white, mean age 67.2 years at symptom onset) tested positive and 67 patients (82.1 % male, 94.0 % white, mean age 60.8 years at symptom onset) tested negative. Further patient characteristics are summarized in table 1. Full LASSO models selected 16 disease features and performed well (AUC 0.91, 0.92 at symptom onset and time of testing respectively). The 5- and single-variable models performed less well in nested cross-validation (AUC 0.84, 0.78 respectively) (Figure 1). The disease features utilized by each model are listed under table 2.
Conclusion: Predictive models for a diagnosis of VEXAS syndrome perform well among patients with systemic inflammatory disease undergoing UBA1 testing. While the full (16-feature) LASSO model performed optimally, the 5-feature model still had satisfactory discrimination and may be considered as a potential clinically applicable tool to guide VEXAS screening. The single variable (monocyte count) model performed less well than the multi-feature models but its discriminatory capacity highlights the importance of low monocytes among patients with systemic inflammation and its association with positive UBA1 testing. Validation of these models in additional VEXAS cohorts is needed to confirm findings.
To cite this abstract in AMA style:
Montes D, Hanson A, Langenfeld H, Crowson C, Patnaik M, Go R, Hines A, Kalantari K, Kusne Y, Lasho T, Mangaonkar A, Olteanu H, Reichard K, Sullivan M, Viswanatha D, Warrington K, Koster M. Developing Predictive Models for the Diagnosis of VEXAS Syndrome [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/developing-predictive-models-for-the-diagnosis-of-vexas-syndrome/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/developing-predictive-models-for-the-diagnosis-of-vexas-syndrome/