Session Information
Date: Tuesday, November 14, 2023
Title: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment: SpA Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: As 41% of patients with psoriasis may be undiagnosed for psoriatic arthritis, treatments must relieve both dermatologic and musculoskeletal symptoms. Deucravacitinib (DEUC), an oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor, is approved in the US, EU, and other countries for treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy. In the pivotal phase 3, randomized, controlled POETYK PSO-1 and PSO-2 trials, significantly greater proportions of patients receiving DEUC achieved 75% improvement from baseline in Psoriasis Area and Severity Index scores and static Physician Global Assessment scores of 0/1 at Week 16 vs patients receiving placebo (PBO) or apremilast (APR). The efficacy and safety of deucravacitinib in patients with active psoriatic arthritis was demonstrated in a phase 2 trial (NCT03881059), with significant improvements vs PBO observed in all key musculoskeletal, functional, and dermatologic outcomes. This analysis compared the effects of DEUC vs PBO and vs APR on peripheral joint disease, joint pain, and health-related quality of life using the 36-item Short Form (SF-36) physical component summary (PCS) score at Weeks 16 and 24 in patients from POETYK PSO-1 and PSO-2 who self-reported joint symptoms.
Methods: POETYK PSO-1 and PSO-2 randomized patients with moderate to severe psoriasis 1:2:1 to PBO, DEUC 6 mg once daily, or APR 30 mg twice daily. The self-administered Psoriatic Arthritis Screening and Evaluation (PASE) questionnaire (≥47 indicates psoriatic arthritis [PASE positive]) was completed by patients with peripheral joint complaints at baseline. Peripheral joint pain and joint disease activity were measured by a visual analog scale (VAS; range, 0–100), with changes from baseline VAS scores assessed only in patients with a baseline of ≥30 for pain and disease. All patients completed the SF-36. Higher scores indicate worse disease burden on VAS measures and better health-related quality of life on the SF-36 PCS.
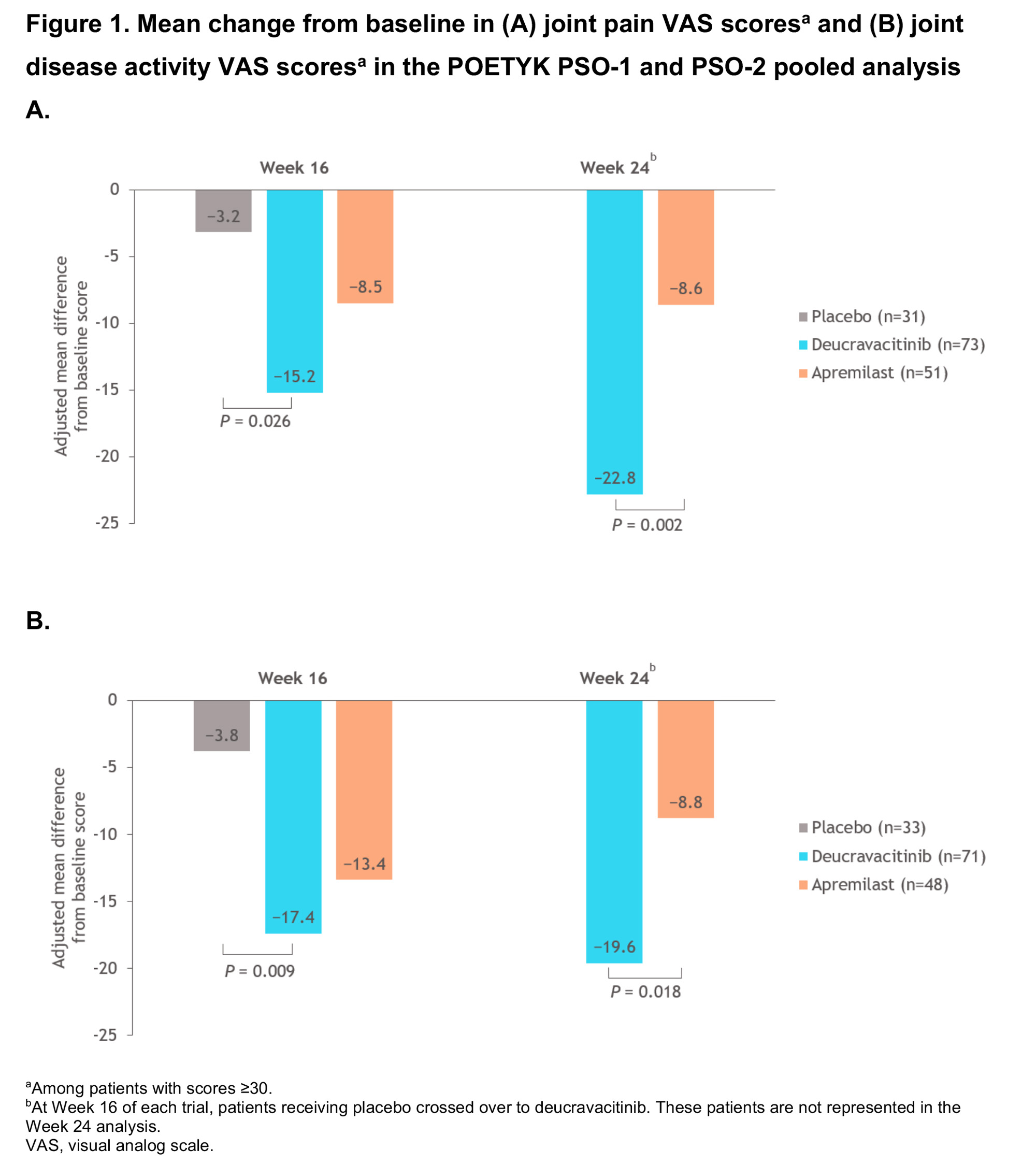
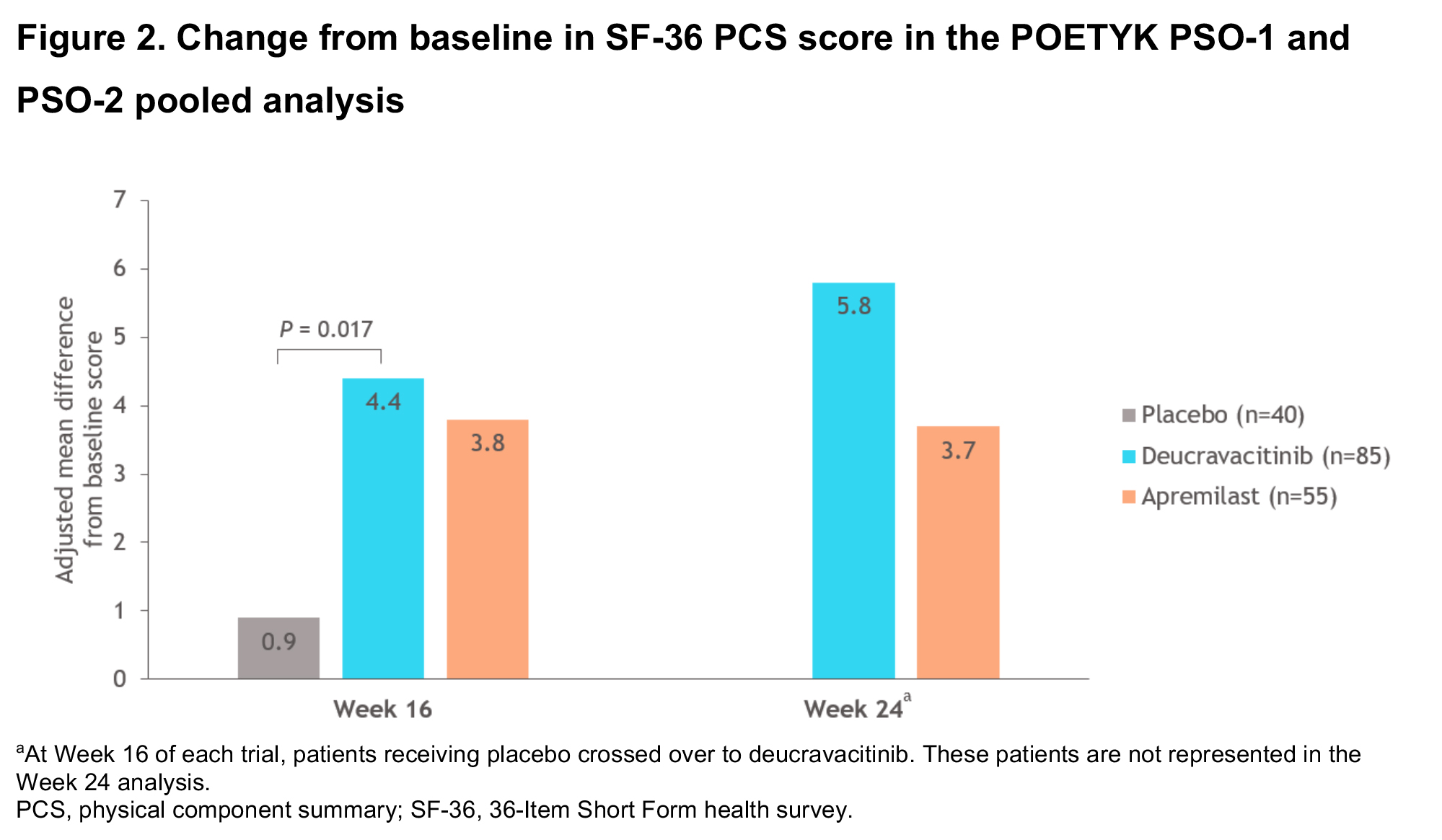
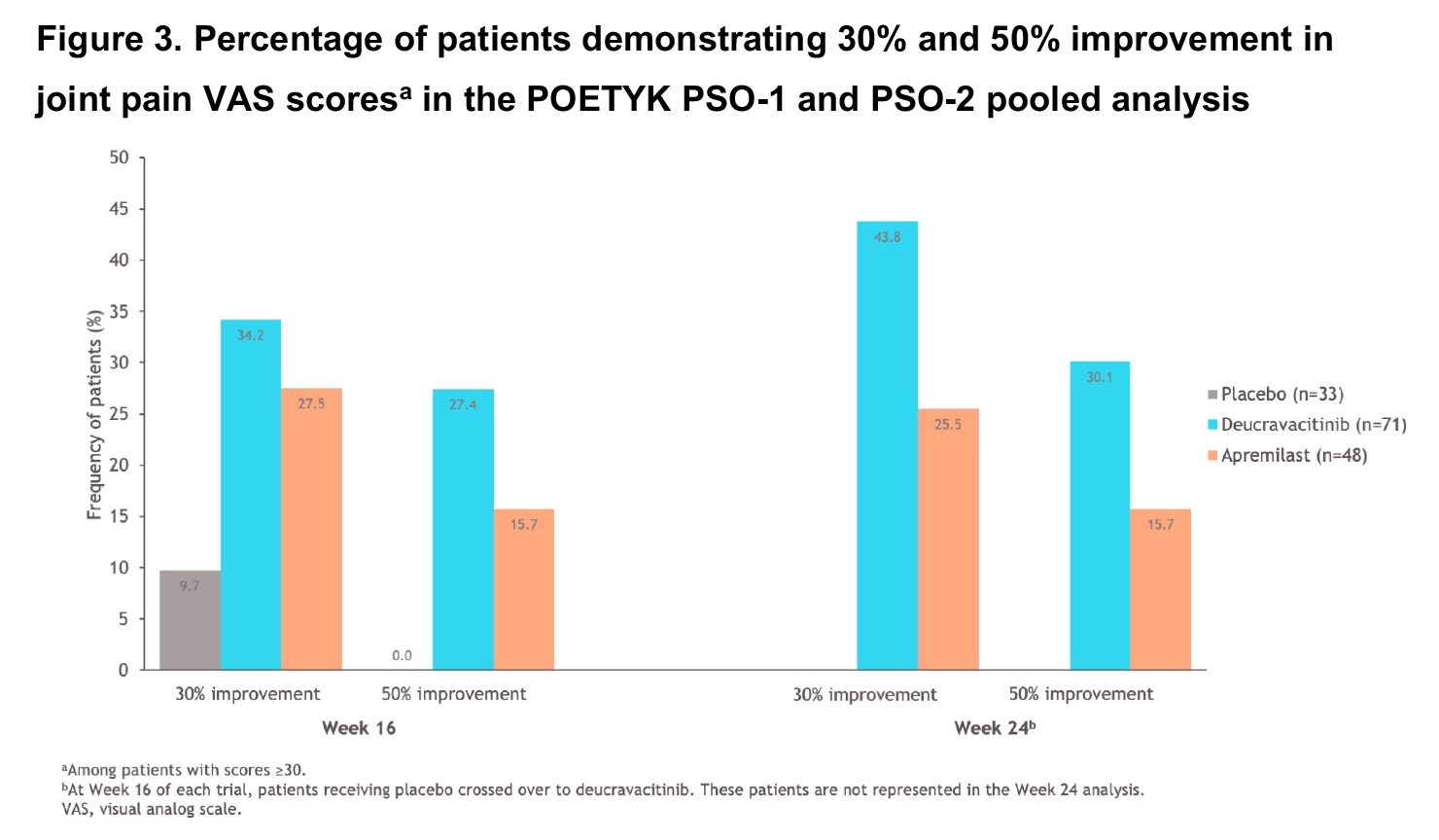
Results: This pooled analysis included 185 PASE-positive patients (11% of 1686 patients in the combined POETYK PSO-1 and PSO-2 trials). The improvement, assessed by adjusted mean change from baseline (CFB), was greater in patients treated with deucravacitinib vs placebo at Week 16 for joint pain VAS, joint disease VAS, and SF-36 PCS scores. Adjusted mean CFBs were greater in patients treated with deucravacitinib at Week 24 vs apremilast for joint pain VAS and joint disease VASscores, and were similar for SF-36 PCS scores (Figures 1, 2). A greater percentage of patients treated with DEUC reported 30% and 50% improvements on the joint pain VAS at Week 16 vs APR and PBO, and at Week 24 vs APR (Figure 3).
Conclusion: PASE-positive patients in POETYK PSO-1 and PSO-2 treated with DEUC reported greater improvements in the impact of joint disease and joint pain vs APR and PBO, and in SF-36 PCS scores vs those receiving PBO. Additionally, a greater percentage of patients treated with DEUC reported 30% and 50% improvements in joint pain vs patients receiving APR and patients receiving PBO. The magnitude of effect among DEUC-treated patients appeared to continue to improve through the 24-week active-controlled period.
To cite this abstract in AMA style:
Merola J, Mease P, Armstrong A, Strand V, Lehman T, Choi J, Becker B, Zhong Y, Colombo M, Thaçi D, Bili A, Gottlieb A. Deucravacitinib, an Oral, Allosteric, Selective Tyrosine Kinase 2 Inhibitor, in Patients with Plaque Psoriasis Who Screened Positive for Psoriatic Arthritis in POETYK PSO-1 and POETYK PSO-2: Effect on Joint Pain and Peripheral Joint Disease vs Placebo and Apremilast [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/deucravacitinib-an-oral-allosteric-selective-tyrosine-kinase-2-inhibitor-in-patients-with-plaque-psoriasis-who-screened-positive-for-psoriatic-arthritis-in-poetyk-pso-1-and-poetyk-pso-2-effect-on/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deucravacitinib-an-oral-allosteric-selective-tyrosine-kinase-2-inhibitor-in-patients-with-plaque-psoriasis-who-screened-positive-for-psoriatic-arthritis-in-poetyk-pso-1-and-poetyk-pso-2-effect-on/