Session Information
Date: Saturday, November 16, 2024
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Deucravacitinib is a first-in-class, oral, selective, allosteric tyrosine kinase (TYK2) inhibitor approved in multiple countries for the treatment of adults with moderate to severe plaque psoriasis. Deucravacitinib selectively inhibits TYK2-mediated signaling of certain cytokines, such as type I interferons, involved in SLE. In the 48-week, double-blind, phase 2 PAISLEY trial (NCT03252587) of deucravacitinib in patients with active SLE, the primary endpoint and all key secondary endpoints were met with the deucravacitinib 3-mg twice-daily (BID) dose, which demonstrated superiority to placebo for the primary endpoint of SLE Responder Index-4 (SRI[4]) response at week 32 and all secondary endpoints at week 48. The aim of this post hoc analysis was to evaluate the efficacy of deucravacitinib 3 mg BID vs placebo at week 48 by baseline demographics and disease characteristics in subgroups of patients treated in the PAISLEY trial.
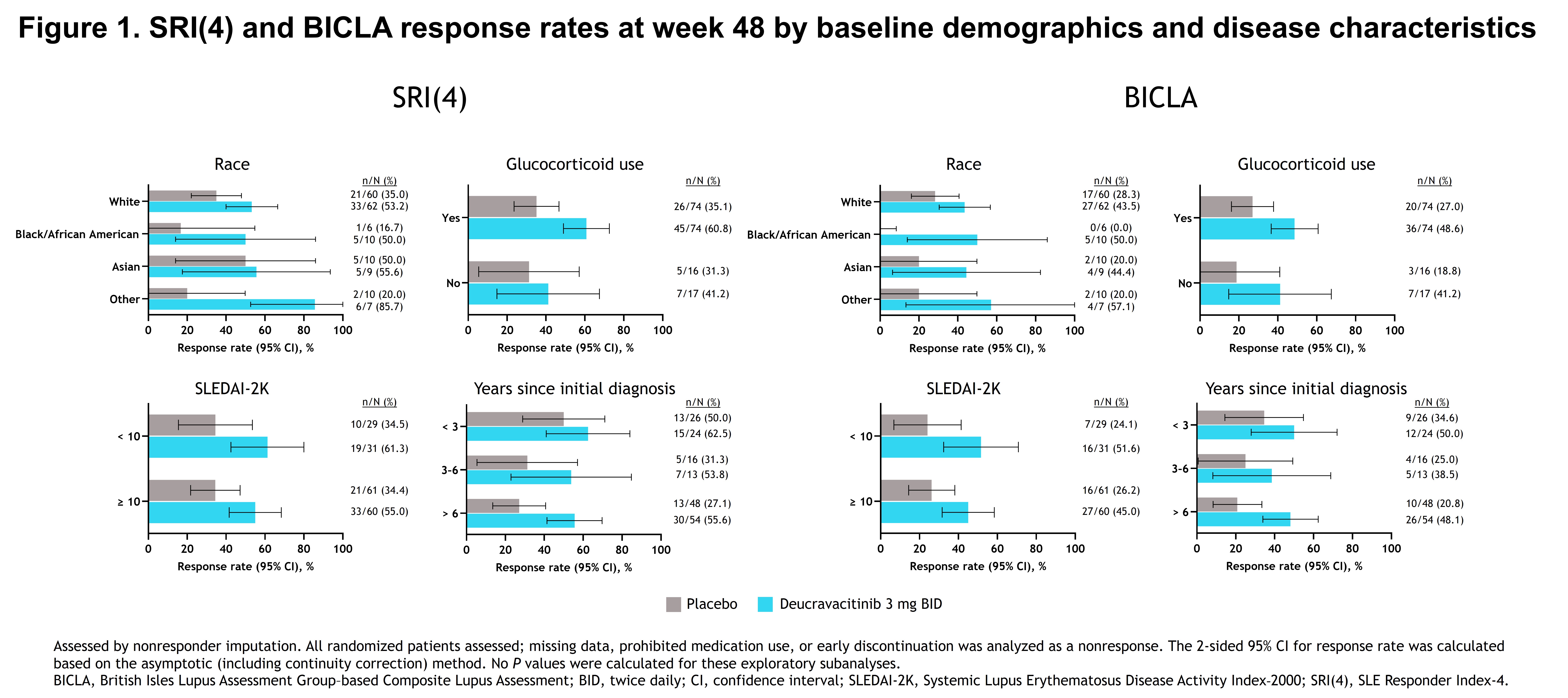
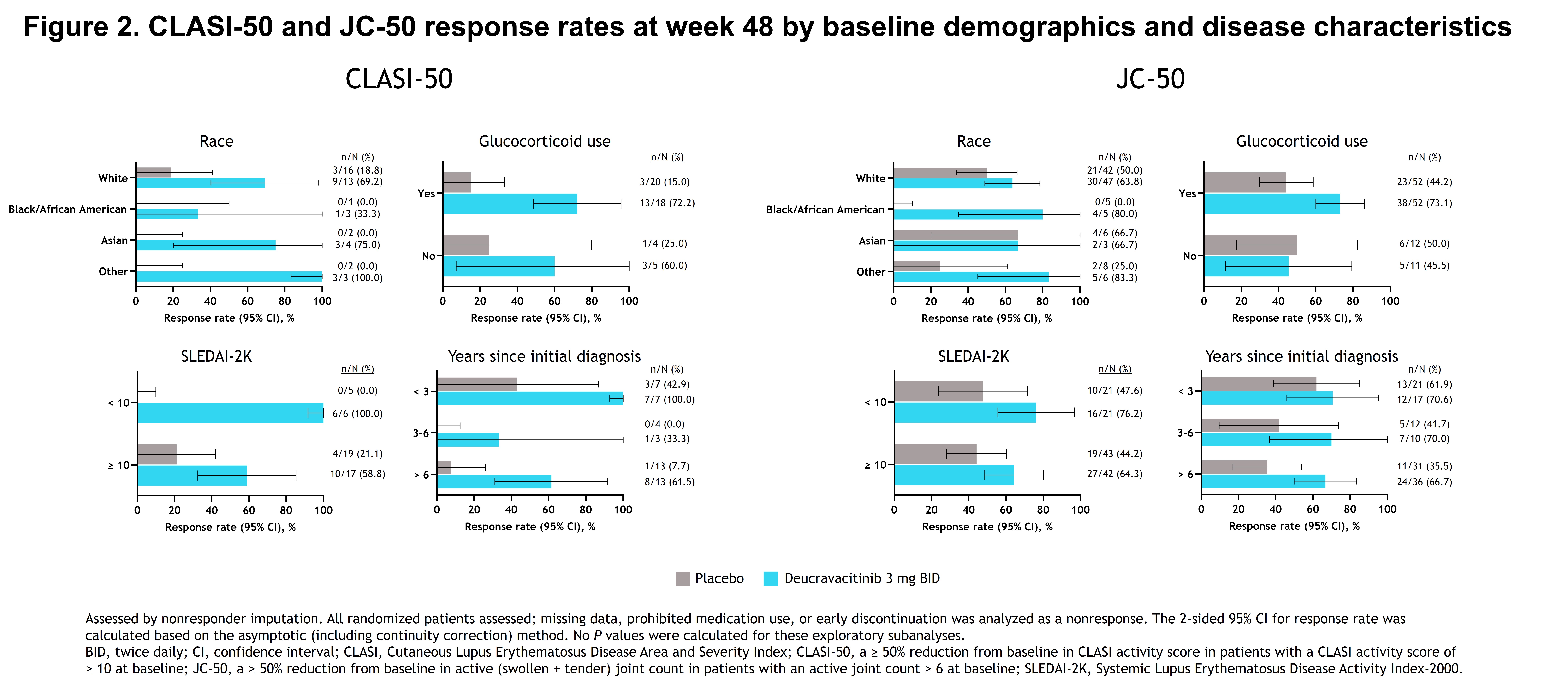
Methods: Patients with active SLE were randomized 1:1:1:1 to placebo (n = 90) or deucravacitinib 3 mg BID (n = 91), 6 mg BID (n = 93), or 12 mg once daily (n = 89). Key secondary endpoints at week 48 were analyzed according to baseline demographics and disease characteristics, including race, baseline glucocorticoid (GC) use, baseline SLE Disease Activity Index-2000 (SLEDAI-2K) score, and disease duration. Study endpoints included SRI(4) response, British Isles Lupus Assessment Group–based Composite Lupus Assessment (BICLA) response, a ≥ 50% reduction from baseline in Cutaneous Lupus Erythematosus Disease Area and Severity Index activity score (CLASI‐50) in patients with a CLASI activity score of ≥ 10 at baseline, and a ≥ 50% reduction from baseline in active (swollen plus tender) joint count (JC-50) in patients with an active joint count of ≥ 6 at baseline. The deucravacitinib 3-mg BID dose generated the optimal benefit-risk profile vs placebo; therefore, for this post hoc analysis, the 3-mg BID dose was determined to be representative of the efficacy of the drug. Response rates were reported with a prespecified nonresponder imputation method for missing values or patients who took prespecified prohibited medications. All analyses were descriptive.
Results: Baseline demographics and disease characteristics were well balanced between the deucravacitinib and placebo arms. Overall, SRI(4) and BICLA response rates increased with deucravacitinib 3 mg BID vs placebo at week 48, regardless of patient race, GC use at baseline, baseline SLEDAI-2K score, or disease duration (Figure 1). Similarly, CLASI-50 and JC-50 response rates increased with deucravacitinib 3 mg BID vs placebo in these subgroups (Figure 2). Data interpretation for some subgroups was limited by low patient numbers.
Conclusion: In the phase 2 PAISLEY trial, deucravacitinib 3 mg BID was associated with improved SRI(4), BICLA, CLASI‐50, and JC-50 response rates at week 48 in multiple patient subgroups with select baseline demographics and disease characteristics. These findings will be validated in the ongoing phase 3 trials of deucravacitinib in SLE.
To cite this abstract in AMA style:
Morand E, Arriens C, Geraldino L, Clarke A, Pomponi S, Hobar C, Wegman T, Koti R, Banerjee S, Van Vollenhoven R. Deucravacitinib, a First-in-Class, Oral, Selective, Allosteric Tyrosine Kinase 2 Inhibitor, in SLE: Efficacy by Baseline Demographics and Disease Characteristics in the Phase 2 PAISLEY Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/deucravacitinib-a-first-in-class-oral-selective-allosteric-tyrosine-kinase-2-inhibitor-in-sle-efficacy-by-baseline-demographics-and-disease-characteristics-in-the-phase-2-paisley-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deucravacitinib-a-first-in-class-oral-selective-allosteric-tyrosine-kinase-2-inhibitor-in-sle-efficacy-by-baseline-demographics-and-disease-characteristics-in-the-phase-2-paisley-trial/