Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: JAK inhibitors have been reported to be a promising treatment for dermatomyositis, an idiopathic inflammatory myopathy. However, the FDA recently added a black box label warning for all JAK inhibitors due to reports indicating increased risk of cardiovascular events, strokes, and cancer. Given these new safety concerns, this study leverages a large North American myositis registry to investigate safety and efficacy outcomes in dermatomyositis patients treated with JAK inhibitors.
Methods: We conducted a retrospective study of adult IIM patients meeting ACR/EULAR criteria for dermatomyositis who have ever received a JAK-inhibitor, tofacitinib, for treatment. We also included a convenience sample of 20 disease controls on alternative immunosuppressive monotherapy to compare safety and efficacy. Each patient chart was reviewed to identify clinical phenotypes and adverse events including cardiovascular events, stroke, cancer and blood clots. Response to treatment was evaluated from clinician recorded muscle strength as defined by the Manual Muscle Testing 8 (MMT-8) score and skin activity as defined by the Cutaneous Disease Area and Severity Index (CDASI) before and after treatment. Differences in clinical characteristics of dermatomyositis patients on tofacitinib vs. alternative monotherapy were assessed by student’s t-test and Fischer’s exact test.
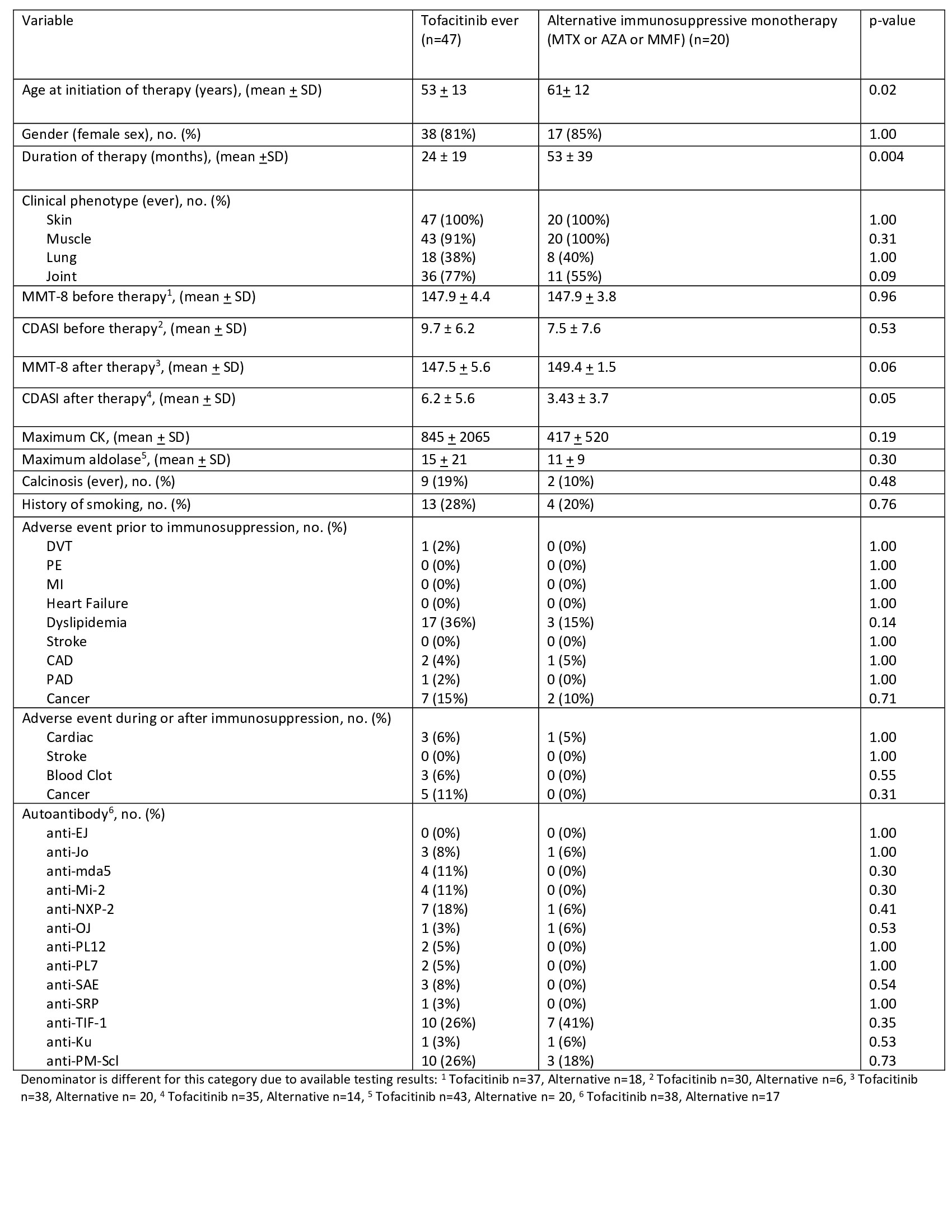
Results: Of the 1800 patients meeting ACR/EULAR criteria for IIM, we identified 47 patients with dermatomyositis ever on tofacitinib. The dermatomyositis patients on tofacitinib were younger (53 ± 13 vs 61 ± 12 years, p=0.02) and had a shorter duration of therapy (24 ± 19 vs 53 ± 39 months, p=0.004) compared to disease controls. Both groups had a similar clinical phenotype of ever skin, muscle, joint, and lung involvement (see Table 1). The most common myositis antibodies in both groups were anti-PM-Scl and anti-TIF-1. MMT-8 at initiation of therapy was 147.9 ± 4.4 in those on tofacitinib vs. 147.9 ± 3.8 in the control group (p=0.96). CDASI at initiation of initiation of therapy was 9.7 ± 6.2 in those on tofacitinib vs. 7.5 ± 7.6 in the control group (p=0.53). In the tofacitinib group, there was a statistically significant difference in CDASI before and after treatment (p=0.02).
Both groups had similar medical histories for clots (1% vs 0% control, p= 1.00), cardiovascular events (10% vs 5% control, p=0.23), and cancer (15% vs 10% control, p=0.71) prior to starting therapy. After a mean duration of therapy of 24 months in the tofacitinib group, there was no significant difference in adverse events with the control group, including cardiovascular outcomes (6% vs 5% control, p= 1.00), strokes (0% vs 0% control, p= 1.000), blood clots (6% vs 0% control, p= 0.55), and cancer diagnosis (11% vs 0% control, p= 0.31).
Conclusion: Tofacitinib may be a valuable therapeutic option for patients with refractory skin predominant dermatomyositis. This study did not show any increased risk of adverse events in comparison to other monotherapies. Limitations include a limited convenience sample that is not controlled for age or duration of therapy. Larger observational studies with longer follow-up time are needed to further assess the safety of tofacitinib in dermatomyositis.
To cite this abstract in AMA style:
Rangaswamy S, Christopher-Stine L, Albayda J, Tiniakou E, Mecoli C, Adler B, Eline E, Kelly W, Paik J. Determining the Long-term Safety and Efficacy of JAK Inhibitors in the Treatment of Dermatomyositis: A Retrospective Cohort Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/determining-the-long-term-safety-and-efficacy-of-jak-inhibitors-in-the-treatment-of-dermatomyositis-a-retrospective-cohort-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/determining-the-long-term-safety-and-efficacy-of-jak-inhibitors-in-the-treatment-of-dermatomyositis-a-retrospective-cohort-study/