Session Information
Date: Sunday, October 26, 2025
Title: (0671–0710) Systemic Sclerosis & Related Disorders – Clinical Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic sclerosis (SSc) is a complex autoimmune condition characterized by vascular abnormalities, immune dysregulation, and progressive fibrosis affecting both the skin and internal organs. Among its manifestations, facial and perioral involvement may lead to mouth opening reduction, known as microstomia, which negatively impacts nutrition, oral hygiene, and the tolerance of procedures like endoscopy or intubation. Despite its clinical impact, it remains unknown who will develop microstomia, and whether it is predictive of other SSc complications. This study aims to determine the evolution of microstomia over time, identify predictors of its progression, and explore its potential association with systemic manifestations of SSc.
Methods: We conducted a longitudinal analysis in the GENISOS cohort, including SSc patients with serial measurements of maximal mouth opening (MO), defined as the distance between the upper and lower lips. The percentage reduction in mouth opening was calculated from baseline to the minimum value recorded for each patient. Only patients with at least two observations separated by at least 6 months were included. Based on the distribution of MO percentage reduction values, a cut-off of 12.3% from baseline (top third of patients with the most significant MO change) was used a priori to define severe microstomia. Patients with a reduction >12.3% were classified as severe (group 2) and compared to those with a reduction 12.3% (group 1). Clinical variables, including disease duration, skin involvement, and symptoms of internal organ dysfunction (e.g., dysphagia), were compared across groups. Logistic and Cox regression models were used to identify predictors of severely reduced MO.
Results: Patients with mild-to-moderate MO reduction (group 1) reached their lowest MO after a median of 0 months from baseline (IQR: 0.0-1.0), while those in the severe group (group 2) reached their lowest point after a median of 1.9 years (IQR: 0.9- 4.15). The severe group had a longer disease duration and a higher prevalence of facial telangiectasia and xerostomia. GI involvement was significantly more prevalent in group 2, with symptoms such as dysphagia, peptic ulcers, diarrhea, bloating, and malabsorption being particularly frequent (Table 1) (Figure 1). Multivariate analysis showed that longer disease duration (OR 1.2 per year; 95% CI 1.1–1.3) and GI Severity Score >1 (OR 2.7; 95% CI 1.1–6.5) were independently associated with severe reduction in MO. Cox regression confirmed that baseline GI involvement (GI Severity Score >1) predicted severe MO reduction ( >12.3%) (HR 2.3; 95% CI 1.8–3.0), with baseline dysphagia (HR:1.4; 95% CI 1.2–1.7) and/or diarrhea (HR:1.8; 95% CI 1.5–2.2) being the strongest predictors (Table 2).
Conclusion: Early GI symptoms—particularly dysphagia and diarrhea—were significantly associated with severe microstomia, suggesting that microstomia may serve as an external marker of progressive internal GI involvement. These findings highlight a potential shared pathophysiological mechanism and underscore the need for further studies to validate this association and elucidate the underlying connection.
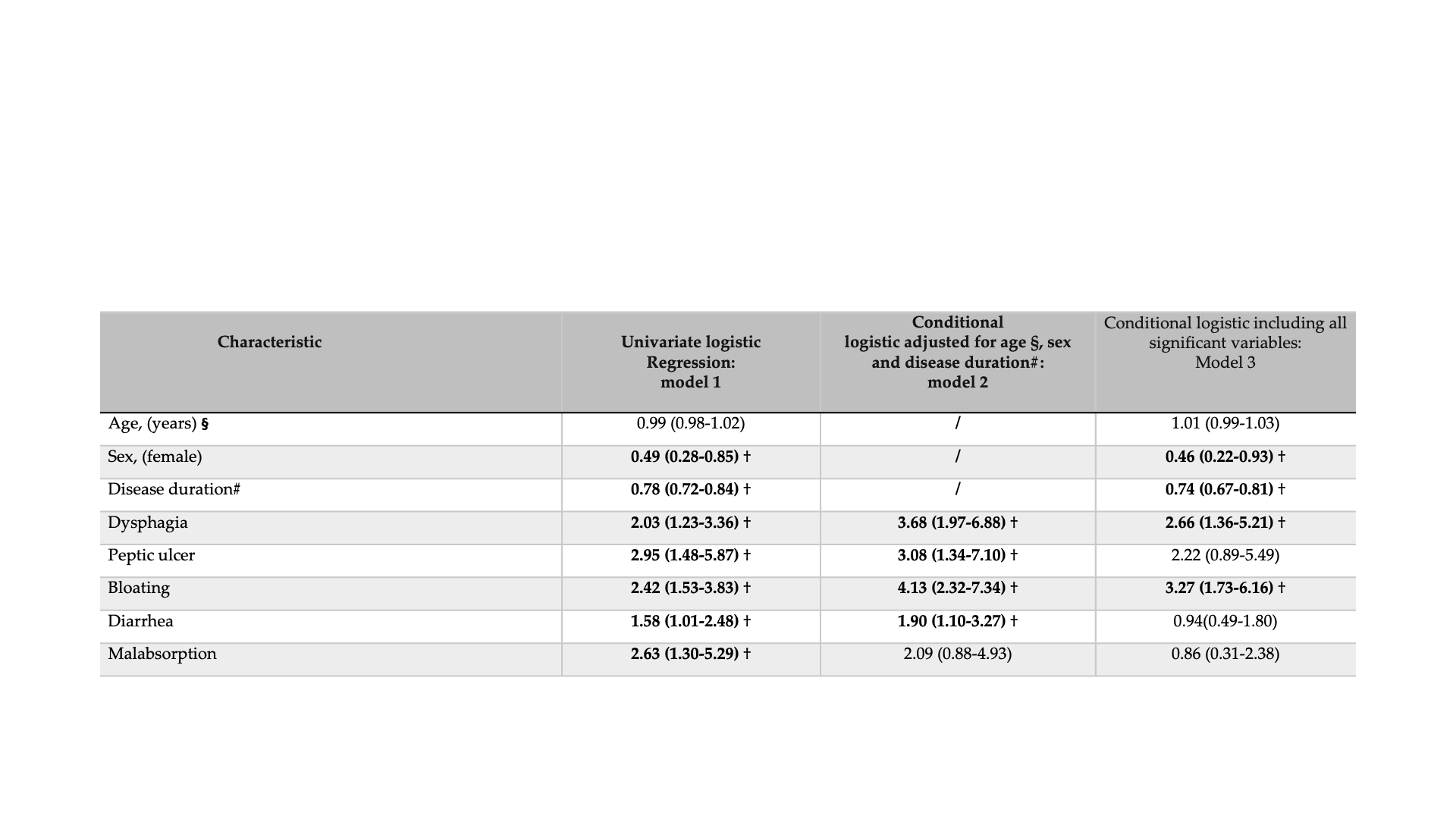 Table1. Longitudinal Comparison of Demographic and Clinical Characteristics in GENISOS SSc Patients With (Group 2) and Without (Group 1) Severe Reduction in Oral Aperture.
Table1. Longitudinal Comparison of Demographic and Clinical Characteristics in GENISOS SSc Patients With (Group 2) and Without (Group 1) Severe Reduction in Oral Aperture.
MO= mouth opening;SD standard deviation; IQR = interquartile range; FVC = forced vital capacity; DLco =diffusing capacity for carbon monoxide; p p-value; GERD = gastroesophageal reflux disease; mRSS = modified Rodnan Skin Score
* Age at the date of minimum oral aperture.
§ Disease duration from any symptom to the date of minimum oral aperture.
‡ Maximum Medsger general, GI, skin, lung, peripheral vascular, and muscle severity score of >1; Maximum Medsger cardiac and joint severity score of >1.
# Value is expressed as a percentage.
FVC and DLco are represented by the minimum values ever recorded.
† Statistically significant.
.gif) Table 2. Cox Regression Analysis of Risk Factors for Severe Oral Aperture Reduction in SSc Patients.
Table 2. Cox Regression Analysis of Risk Factors for Severe Oral Aperture Reduction in SSc Patients.
Definition of Gastrointestinal manifestations:(a) Dysphagia, defined as pain or difficulty in swallowing or passing food;(b) Peptic ulcer, documented; (c) Bloating;(d) Diarrhea;(e) Malabsorption, confirmed by diarrhea associated with >10% weight loss or by abnormal chemical studies. Symptoms from (c) to (d) were self-reported by patients.
*It refers to the age at baseline (years).
# It refers to the disease duration from the first non-Raynaud symptom at baseline (years).
§Values are Hazard ratio (95% confidence interval).
† Statistically significant.
.gif) Figure 1. Distribution of maximal percent reduction in oral aperture across worsening Medsger GI Severity Scores.
Figure 1. Distribution of maximal percent reduction in oral aperture across worsening Medsger GI Severity Scores.
Score 0 (n = 52): median 0%, IQR 0–11.55
Score 1 (n = 241): median 6.25%, IQR 0–14.28
Score 2 (n = 31): median 15.55%, IQR 5–24
Score 3–4 (n = 18): median 16.1%, IQR 0–28
The p-value was calculated using the Kruskal-Wallis test to compare differences between groups. P-value < 0.001
To cite this abstract in AMA style:
Di Ciommo F, Balar A, Anderton R, Hughes M, Skaug B, Mayes M, Assassi S, Ayla A, McMahan Z. Determinants of Progressive Microstomia in Systemic Sclerosis: Insights from the GENISOS Cohort with a Focus on GI Involvement [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/determinants-of-progressive-microstomia-in-systemic-sclerosis-insights-from-the-genisos-cohort-with-a-focus-on-gi-involvement/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/determinants-of-progressive-microstomia-in-systemic-sclerosis-insights-from-the-genisos-cohort-with-a-focus-on-gi-involvement/
