Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Measures & Measurement of Healthcare Quality (2615–2620)
Session Type: Abstract Session
Session Time: 3:30PM-3:45PM
Background/Purpose: Patients prescribed immunosuppressive medications are at increased risk for herpes zoster and related complications, and the American College of Rheumatology recommends the recombinant zoster vaccine (Shingrix) for most patients receiving these treatments. However, fewer than half of Veterans in the VHA receiving immunosuppressive therapy have received even one dose of the Shingrix vaccine. To address this gap, we developed a national EHR-based dashboard to promote guideline-concordant Shingrix vaccination across the VHA and deployed it using a wait-list randomized control trial design.
Methods: Using data from the VHA Corporate Data Warehouse, we identified patients eligible for Shingrix according to ACR vaccination guidelines. A PowerBI dashboard was created to display patient-level information (immunosuppressant use, vaccination status) and facility-level vaccination rates (Figure 1).All 131 VHA facilities were invited to participate in a waitlist-randomized trial of dashboard access. Seventy-three facilities responded to the invitation, and they were randomized to either “early access” (September 2024) or “late access” (Summer 2025), stratified by facility size and baseline percentage of patients vaccinated. Dashboard use was tracked using PowerBI user logs. The main outcome was the percentage of eligible patients with at least one dose of Shingrix, calculated weekly between June 2024 and March 2025 (38 weeks). A two-group interrupted time series (ITS) analysis was conducted to compare rates of improvement in early-access vs. late-access (control) sites.
Results: The 73 participating facilities served 79,357 Veterans prescribed immunosuppressive medications at baseline; 87% were age ≥50, 88% male, 73% white, and 89% non-Hispanic. The most common regimens were csDMARD monotherapy (37%) and biologic monotherapy (20%).Most facilities (79%) were high-complexity sites located in the North Atlantic (23%) or Southeast (25%) regions. At the 38 early-access sites, 71% (27/38) had at least one clinician access the dashboard at least once during the study period. Access peaked in the first month after launch and was more sporadic thereafter (median (IQR) number of sessions over the study period: 38 (38 – 114)).In this analysis of the first 38 weeks post-launch, Shingrix vaccination increased modestly from 57.1% to 59.5% across all sites. Early-access facilities saw a post-intervention increase of 0.07 percentage points (pp) per week, compared to 0.05 pp/week at control sites. The difference in trends was not statistically significant (difference: 0.02 pp/week; 95% CI: –0.01 to 0.05) (Table; Figure 2).
Conclusion: We designed an innovative wait-list randomized control trial of a provider-facing immunization dashboard to improve Shingrix vaccination. After 4 months of implementation, we observed no significant difference in Shingrix vaccination rates between early-access and control sites. This may reflect the typical 3–4 month interval between rheumatology visits. Longer follow-up, enhanced promotion of the dashboard, and additional clinician and patient education may be needed to realize the full impact of this intervention.
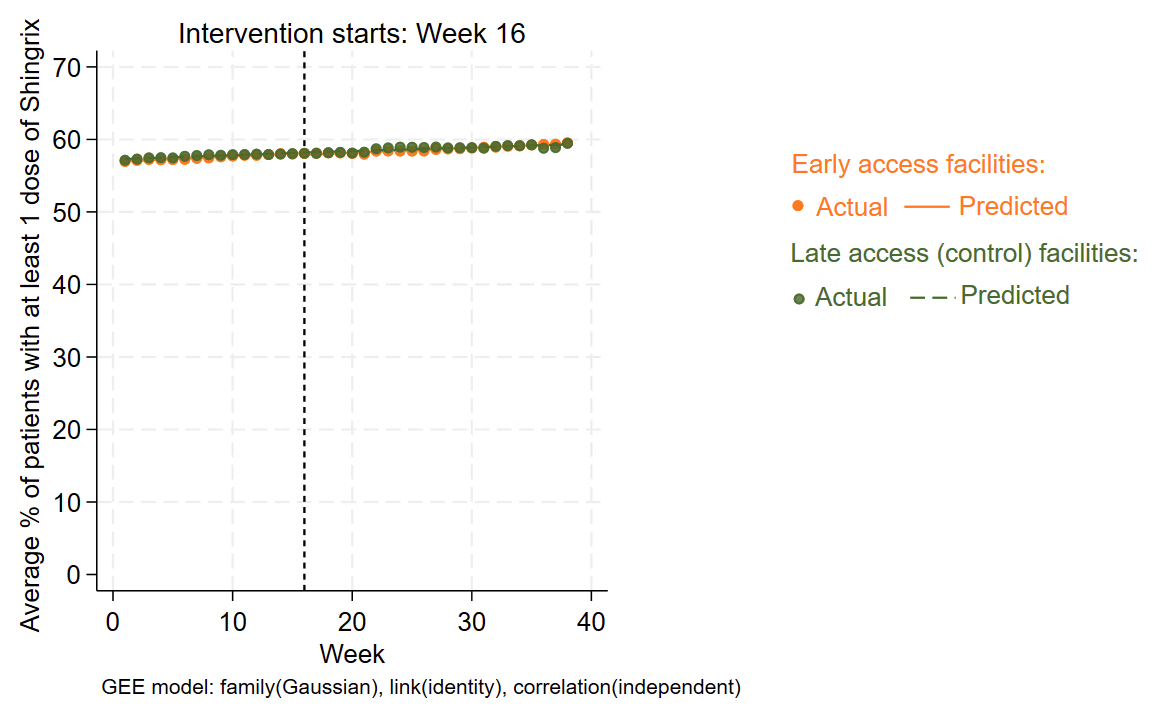 Table. Percentage of patients with at least one Shingrix vaccine, overall and by groups at the beginning and the end of the study period
Table. Percentage of patients with at least one Shingrix vaccine, overall and by groups at the beginning and the end of the study period
.jpg) Figure 1. Screenshot of the Shingrix immunization dashboard (fictitious data)
Figure 1. Screenshot of the Shingrix immunization dashboard (fictitious data)
.jpg) Figure 2. Interrupted time series (ITS) analysis assessing Shingrix vaccination pre- and post-dashboard access for “early access” versus “late access” (control) facilities
Figure 2. Interrupted time series (ITS) analysis assessing Shingrix vaccination pre- and post-dashboard access for “early access” versus “late access” (control) facilities
To cite this abstract in AMA style:
Li J, Wilson C, Tarasovsky G, Kwon S, Whooley M, Schmajuk g. Design and Initial Results from a Cluster Randomized Trial of a Provider-Facing Immunization Dashboard to Improve Shingrix Vaccination in the Veterans Health Administration [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/design-and-initial-results-from-a-cluster-randomized-trial-of-a-provider-facing-immunization-dashboard-to-improve-shingrix-vaccination-in-the-veterans-health-administration/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/design-and-initial-results-from-a-cluster-randomized-trial-of-a-provider-facing-immunization-dashboard-to-improve-shingrix-vaccination-in-the-veterans-health-administration/
