Session Information
Date: Sunday, November 12, 2023
Title: (0176–0195) Healthcare Disparities in Rheumatology Poster I: Lupus
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Participation in clinical trials is part of treatment for many patients with chronic diseases. However, patients with systemic lupus erythematosus (SLE), especially those of African American and Hispanic descent, have been reluctant to participate in clinical trials. Qualitative research identified patient, provider, community, and study design factors as the main reasons for this hesitancy. Concerted efforts to increase awareness of and engagement in SLE clinical trials are underway. We evaluated factors associated with challenges to clinical research enrollment in the Columbia University (CU) and Albert Einstein College of Medicine (AECOM) lupus cohorts in New York City (NYC) that serve the low socioeconomic status communities of Washington Heights and the Bronx.
Methods: This study sponsored by the U.S. Department of Health and Human Services, Office of Minority Health, plans to enroll 200 patients in an engagement program modeled after the Lupus Research Alliance Patient Advocates for Lupus Studies (PALS) program. SLE patients were invited to participate during routine clinic visits. Patients were apprised of the study in detail and their decision to participate or refuse was recorded. Socio-demographics and disease characteristics were collected. Data from recent therapeutic clinical trial participants was included for comparison. One-way ANOVA was used to detect differences among the 3 groups: enrolled in the study, refused participation and clinical trial participants.
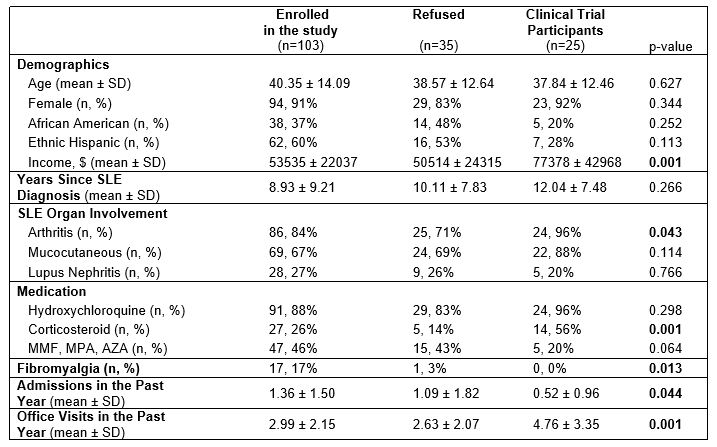
Results: Of the 138 patients asked to participate, 103 (74.6%) agreed while 35 (25.4%) refused. Additionally, 25 clinical trial participants were included. Participants enrolled in the educational sessions, demonstrating willingness to engage in clinical trial education, were more likely to have been admitted during the past year (1.36 vs 1.09 vs 0.52, p=0.044) and have co-morbid fibromyalgia (17% vs 3% vs 0%, p=0.013). While there were more African American and Hispanic patients in the education study groups, these differences did not reach statistical significance. Detailed data is summarized in Table 1. The major reasons for refusal were lack of interest in clinical trials (17, 49%), time constraints (14, 40%) and negative prior experiences relating to clinical trials (4, 11%). Clinical trial participants were more likely to have arthritis (84% vs 71% vs 96%, p=0.043), mucocutaneous manifestations (67% vs 69% vs 88%, p=NS) and be on steroids (26% vs 14% vs 56%, p=0.001) as required for inclusion in clinical trials. Also, the clinical trial group displayed a higher zip-code median income (54K vs 51K vs 77K, p=0.001) and more rheumatology office visits in the past year (2.99 vs 2.63 vs 4.76, p=0.001).
Conclusion: These data suggest that people’s intention to participate in clinical research is influenced by disease severity (admissions and office visits), patient factors (income and co-morbid fibromyalgia) and study design (arthritis, steroid use). It is difficult to ascertain if racial and ethnic factors affect the current study enrollment. More data is needed to confirm the role of these factors and additional qualitative data will help identify factors that affect a patient’s decision at the individual level.
To cite this abstract in AMA style:
Inzerillo S, Schwartz N, Khalili L, Fu W, Tang W, Geraldino-Pardilla L, Gartshteyn Y, Schmidt N, Izmirly P, Askanase A. Demographic and Clinical Factors That Contribute to Clinical Study Enrollment in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/demographic-and-clinical-factors-that-contribute-to-clinical-study-enrollment-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/demographic-and-clinical-factors-that-contribute-to-clinical-study-enrollment-in-systemic-lupus-erythematosus/