Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Pediatric antiphospholipid syndrome (APS) is a rare, thrombo-inflammatory autoimmune disease characterized by thrombosis and nonthrombotic manifestations in patients with persistent positive antiphospholipid antibodies, with clinical features that differ from adult APS. Current APS classification criteria are derived from adult data, and pediatric APS data collection is fragmented and inconsistent between institutions and regions. This project aims to develop an international consensus that defines critical data fields to facilitate international registry-based pediatric APS research.
Methods: Data fields were compared from existing International Ped-APS Registry and CARRA Registry, which identifies patients with APS within the lupus cohort. The 2023 ACR/EULAR APS classification criteria were reviewed and systematic literature review of pediatric APS between 2003-2024 was conducted to identify potential data elements for future registries (Figure 1). Delphi surveys were completed by physicians with APS experience, which included pediatric rheumatologists, med/peds rheumatologists, adult rheumatologists, and pediatric hematologists representing 12 countries and two caregiver representatives. Items were scored from 1 (not important) to 9 (very critical). Consensus “in” was reached for core items scored > 7 by > 70% of experts, as well as all items included in the adult 2023 APS Classification Criteria. Consensus “out” was reached for items with average score < 5. The remaining items were labeled “equivocal” for voting at a hybrid consensus meeting. During the meeting, items with >80% agreement were included in the final dataset (either Core or Expanded), and items failing to meet consensus were either excluded or revised and re-evaluated when appropriate. Items scoring >6 were eligible for either Core or Expanded datasets. Items scoring > 5 but < 6 were only voted upon for the Expanded dataset.
Results: Thirty-two Delphi surveys were completed in round 1 and twenty-six in round 2. Two caregiver representatives completed a modified Delphi. Of 316 final candidate items (Figure 2), 72 items reached consensus “in”, 87 items were consensus “out” and 157 “equivocal” items were discussed at the hybrid consensus meeting (Table 1). Nineteen participants voted upon Core and Expanded Datasets for each “equivocal” item. The final dataset included 60 core data elements and 22 expanded data elements, spanning 18 thematic domains.
Conclusion: Through systematic evaluation of existing registries, literature review, and Delphi process, we have identified several critical variables to inform future pediatric APS registries. By leveraging international expertise and integrating it with patient patient perspectives, we are laying a solid foundation for the development of a harmonized dataset to advance pediatric APS research efforts globally.
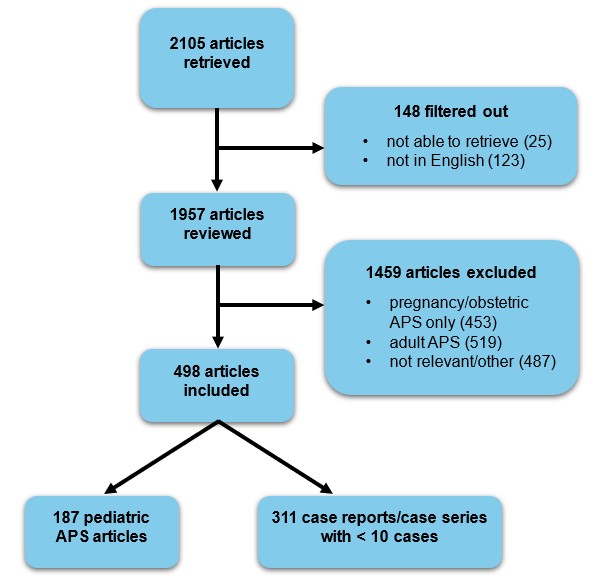 Figure 1. Flow diagram of literature review to identify candidate items
Figure 1. Flow diagram of literature review to identify candidate items
.jpg) Figure 2. Flow diagram showing selection process of candidate items for Delphi survey to identify critical data fields in pediatric APS registries
Figure 2. Flow diagram showing selection process of candidate items for Delphi survey to identify critical data fields in pediatric APS registries
.jpg) Table 1: List of consolidated core and expanded clinical datasets
Table 1: List of consolidated core and expanded clinical datasets
To cite this abstract in AMA style:
Bhatt J, Sloan E, Demir S, Avramovic M, Özen S, Erkan D, Avcin T. Defining a Consensus for Critical Data Fields for International Pediatric Antiphospholipid Syndrome Research [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/defining-a-consensus-for-critical-data-fields-for-international-pediatric-antiphospholipid-syndrome-research/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/defining-a-consensus-for-critical-data-fields-for-international-pediatric-antiphospholipid-syndrome-research/
