Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The Accelerating Medicines Partnership (AMP®) is a public-private partnership with the goal of transforming the current model for developing new diagnostics and treatments by improving understanding of the relevant biological pathways through a deconstruction-reconstruction approach to selected diseases. The goal of the Sjögren’s Team for Accelerated Medicines Partnership (STAMP) is to: 1) understand phenotypic and molecular heterogeneity of SjD, and disease mechanisms inherent to progression of SjD; 2) identify therapeutic targets to stabilize early disease and reverse or improve advanced disease; and 3) understand disease mechanisms and molecular overlap of SjD and SLE or RA.
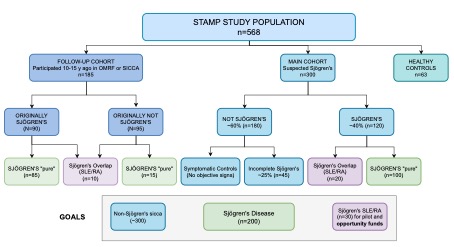
Methods: STAMP reunites investigators from 6 academic institutions assembling a cohort of carefully characterized participants with SjD, non-Sjögren’s sicca, SjD-SLE overlap, and Healthy Volunteers as the source of SjD data and biospecimens for AMP®-AIM. While its study design is cross-sectional, STAMP will include past participants from two previous SjD cohorts, 10-15 years following their initial comprehensive evaluation for SjD. The recruitment strategy and expected distribution within the cohort are shown in Figure 1. All study procedures are conducted under a single IRB mechanism.
Results: To date, the completed STAMP planning and pilot phases have a vast array of deliverables.
Development of a 5-year scientific agenda to 1. Predict SjD development and progression in labial salivary glands (LSG) and systemic disease; 2. Analyze interactions between immune cells and glandular secretory/ductal cells and elucidate SjD molecular and phenotypic heterogeneity using multi-omic data; 3. Investigate the determinants of glandular tropism.
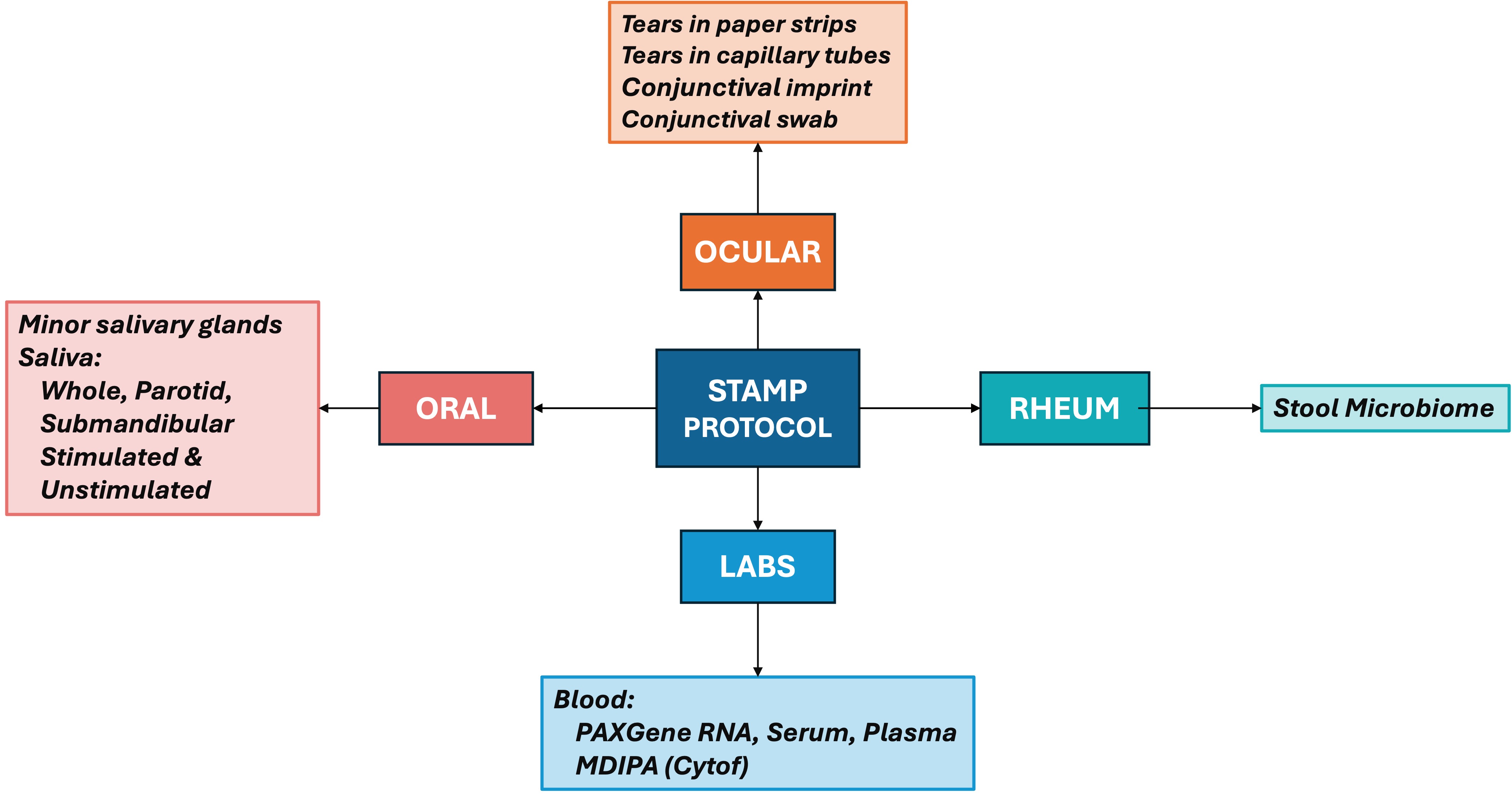
Development and testing of clinical and laboratory SOPs with trans-disease and disease-specific components, and a custom-designed eCRF that captures 1,240 datapoints per participant.
Training and calibration of specialists for the salivary gland ultrasonography, ocular assessments, advanced tissue analysis/imaging, histopathological characterization and reporting based on digital pathology with % agreement of STAMP raters
vs. gold standard ranging from 80 to 95.
Recruitment of 152 participants spanning SjD, non-Sjögren sicca, longitudinal cases, and healthy volunteers.
Tissue and technology pilot studies: 1. Testing and optimization of methods of cryopreservation, dissociation, and fixation of LSG tissues. 2. Feasibility of deploying cutting-edge technologies to assess a) new and 10 to 15-year-old FFPE LSG tissues; b) 10X 5’ and 3’ single cell RNA sequencing (scRNAseq) in fresh and cryopreserved tissues; and c) State-of-the-art Xenium and CosMX Spatial Transcriptomics platforms on FFPE LSG tissues.
Conclusion: After successful completion of the planning and pilot phases, the STAMP team, working with the AMP Network cores, is in the scale-up phase. Public access for external scientists to scRNAseq, spatial transcriptomic, and phenotypic data will be available 6 months after data are collected/generated. Beyond STAMP, the investigators participate in data and technology cores or “Opportunity Fund” projects.
To cite this abstract in AMA style:
Rasmussen A, Farris A, Baer A, Warner B, Lessard C, Shiboski S, Shiboski C. Deconstruction and Reconstruction of Sjögren’s Disease: AMP®-AIM Partnership [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/deconstruction-and-reconstruction-of-sjogrens-disease-amp-aim-partnership/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deconstruction-and-reconstruction-of-sjogrens-disease-amp-aim-partnership/