Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Sjögren’s syndrome (SS) is an autoimmune disease characterized by progressive immune cell-mediated destruction of the exocrine glands. SS patients have distinct clinical phenotypes based on their autoantibody profiles; anti-SSA positive patients often present with hypergammaglobulinemia, more severe glandular dysfunction, and extraglandular manifestations, whereas anti-centromere positive patients show a higher incidence of Raynaud’s phenomenon and sclerodactyly. Despite these clinical differences and the common presence of salivary gland destruction, the underlying pathogenic mechanisms driving salivary gland destruction remain understood.
Methods: To elucidate the complex cellular interplay driving salivary gland inflammation in SS, we performed an integrative analysis using multimodal single-cell technologies including RNA sequencing (scRNA-seq) together with T-cell receptor (TCR) and B-cell receptor (BCR) repertoire analysis on salivary gland samples from SS patients stratified by their autoantibody profiles (SSA+, n=8; CENT+, n=4; SSA+CENT+, n=4; Sicca, n=8). Furthermore, we analyzed spatial transcriptomic data of salivary gland samples from SS patients (SSA+, n=6; CENT+, n=5; SSA+CENT+, n=4; Sicca, n=5) to characterize pathogenic cell types in the inflammation.
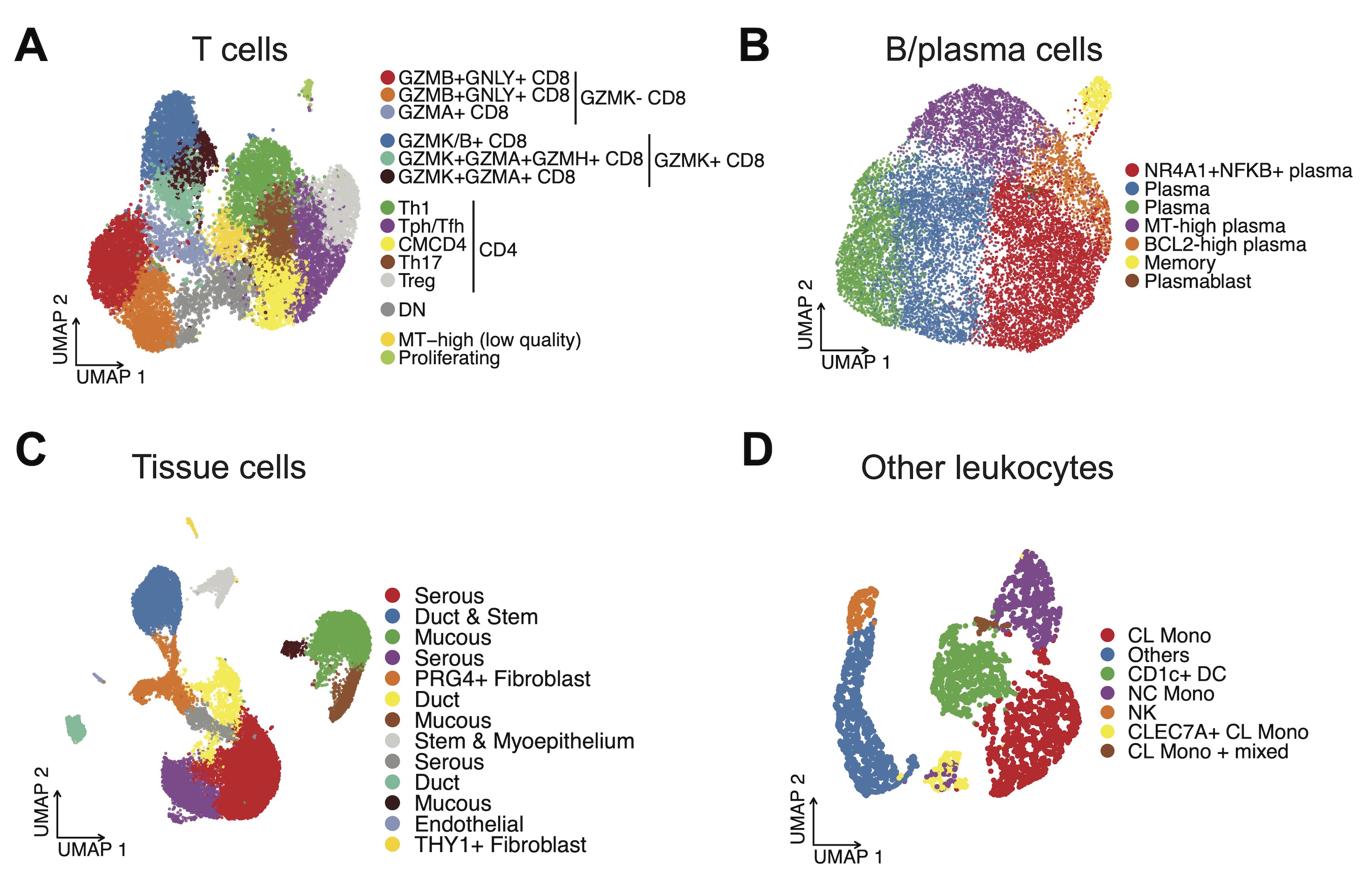
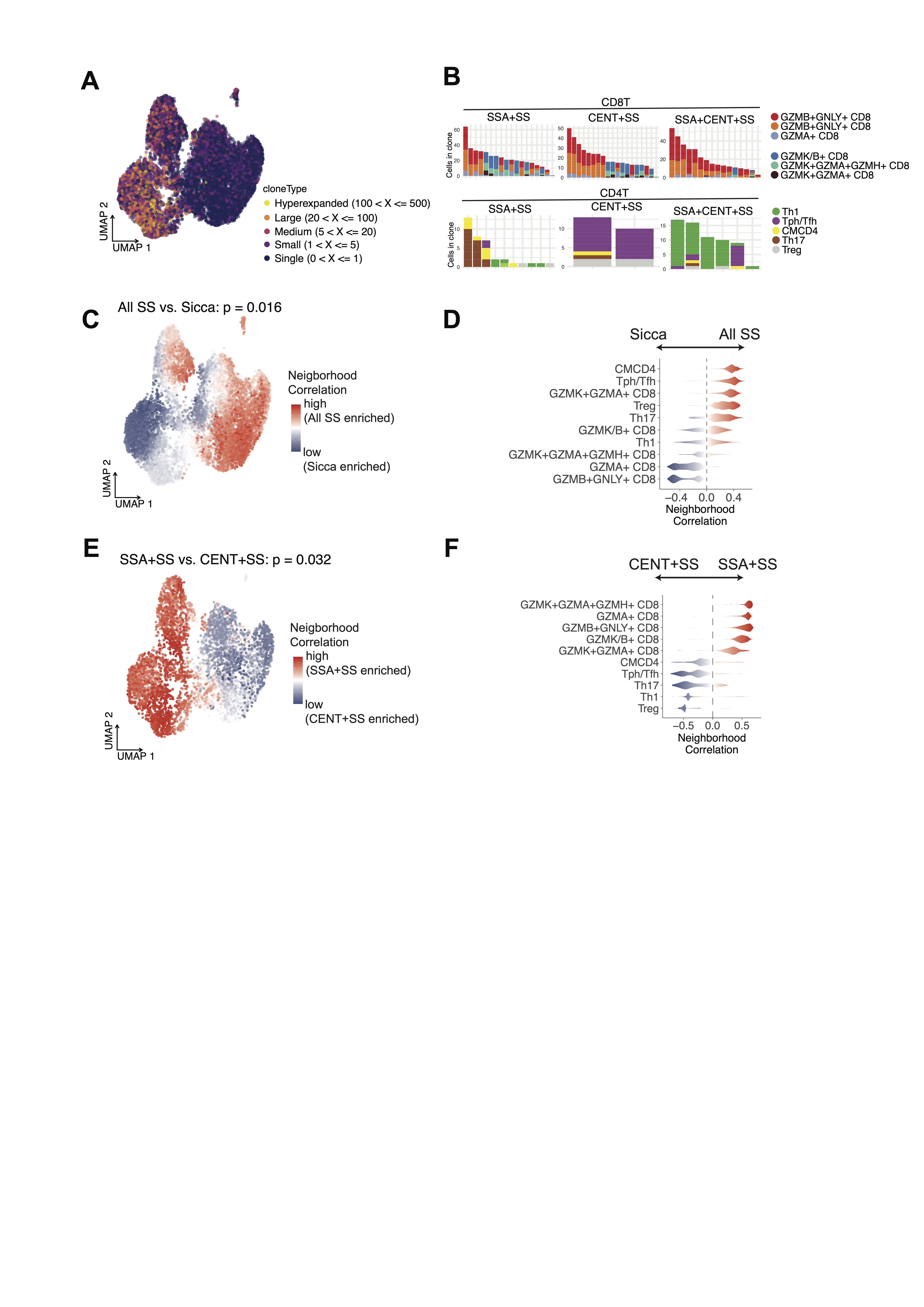
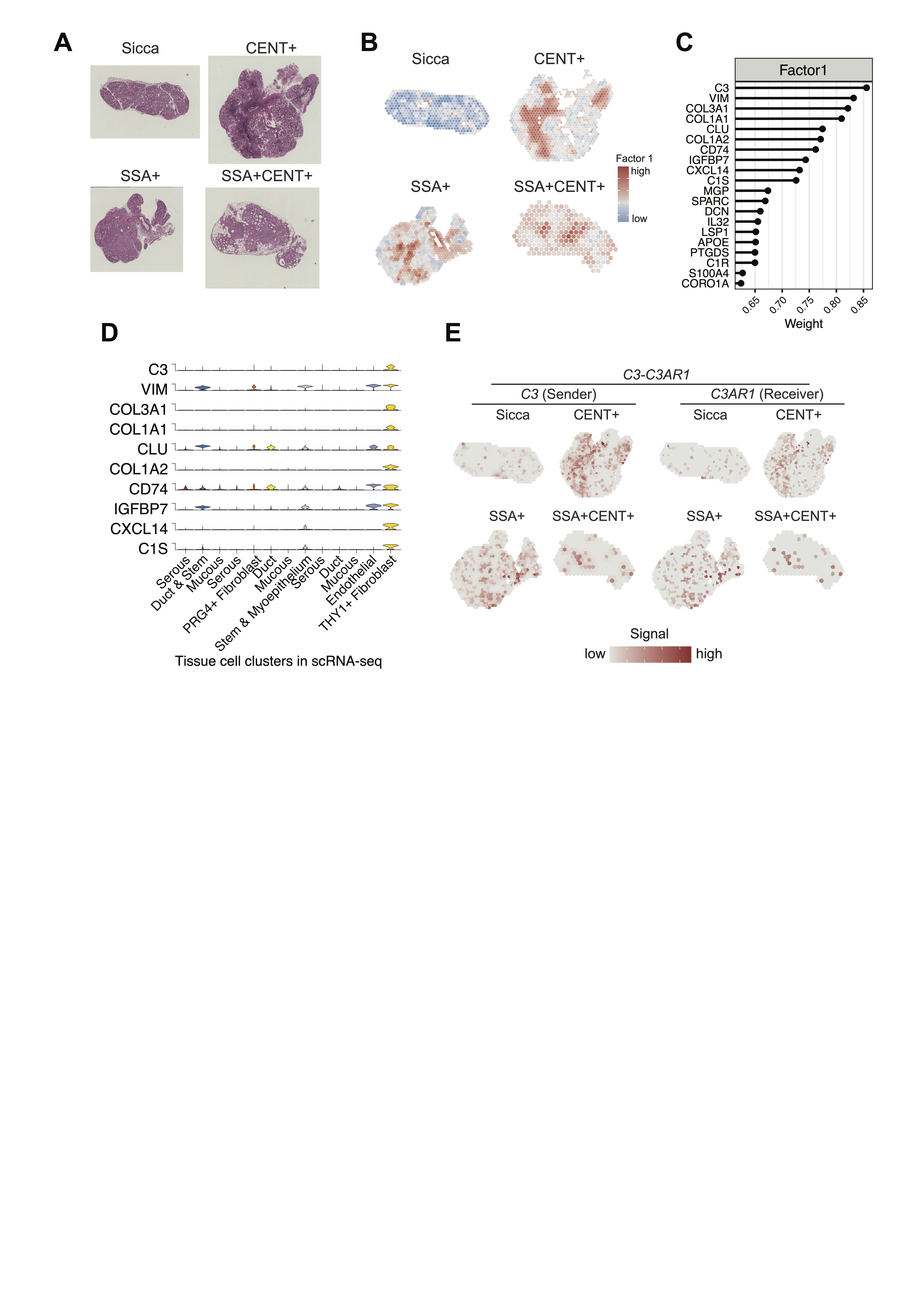
Results: We identified fine-scaled and heterogeneous cell types in salivary glands based on the gene expression pattern (Fig. 1A-D). Within the T cell clusters, GZMB+ and GZMK+ CD8 T cells were clonally expanded independently, regardless of disease status (Fig. 2A-B). In SS samples, increased GZMK+ CD8 T, Tph/Tfh, and Treg were shared features (Fig. 2C-D), with CD8 subsets more common in SSA+SS and CD4 subsets more common in CENT+SS (Fig. 2E-F). Both TCR and BCR diversity was higher in CENT+ phenotypes, reflecting that the autoantigens targeted by anti-centromere antibodies are more diverse than those of anti-SSA antibodies. Furthermore, unsupervised deconstruction analysis of spatial transcriptome data revealed a disease related signature (factor 1) at the focus area (Fig. 3A-B). This signature was composed of complement-mediated cell-cell interactions by inflammatory fibroblasts, which are assumed to amplify inflammation in the salivary gland lesion (Fig. 3C-E).
Conclusion: Our findings provide a deep understanding of the cellular landscape and intercellular communication within the salivary glands, shared and varying by autoantibody profile. This work advances our understanding of how autoimmunity is orchestrated at the cellular level in SS and opens new avenues for the identification of potential therapeutic targets in this complex autoimmune disorder.
To cite this abstract in AMA style:
Inamo J, Takeshita M, Suzuki K, Tsunoda K, Usuda S, Kuramoto J, Takeuchi T, Kaneko Y. Deciphering Salivary Gland Inflammation in Sjögren’s Syndrome Reveals Shared and Autoantibody-Specific Immune Cell Heterogeneity [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/deciphering-salivary-gland-inflammation-in-sjogrens-syndrome-reveals-shared-and-autoantibody-specific-immune-cell-heterogeneity/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deciphering-salivary-gland-inflammation-in-sjogrens-syndrome-reveals-shared-and-autoantibody-specific-immune-cell-heterogeneity/