Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The pivotal role of CD40-CD40L interactions in systemic lupus erythematosus (SLE) pathogenesis stems from the orchestration of a range of immune and inflammatory responses involving B cells, T cells, antigen-presenting cells, and type I interferon (IFN) production.1,2 Dapirolizumab pegol (DZP) is a polyethylene glycol-conjugated antigen-binding fragment lacking a functional Fc domain that inhibits CD40L.3 This post hoc pharmacodynamic analysis explored the impact of DZP on B cell and type I IFN pathways using data from the phase 2b RISE trial in SLE (NCT02804763).3
Methods: In RISE, patients (pts) received placebo (PBO) or DZP (6/24/45 mg/kg) alongside standard of care (SOC) for 24 weeks (wks); adults with moderately to severely active SLE, receiving stable doses of SOC treatments, were included in the trial.3 Analyses presented here used a subgroup of patients from RISE (n=131) that is analogous to the phase 3 trial population(NCT04294667);4 results are shown for the PBO and DZP 24 mg/kg arms. RNA sequencing (RNAseq) was performed on available blood samples at baseline and Wks 2 and 24; samples were not available for all pts at all timepoints. Gene expression changes were analyzed and competitive gene set analyses were carried out on pathways relevant to SLE immunopathology, selected from Gene Ontology Biological Processes and augmented with gene signatures that discriminate immune cell types in SLE.5,6 Differential expression results for DZP treatment were corrected for SOC effects. To assess effects of DZP on type I IFN biology, pts were stratified post hoc by baseline type I IFN expression levels using a 4‑gene type I IFN signature.7
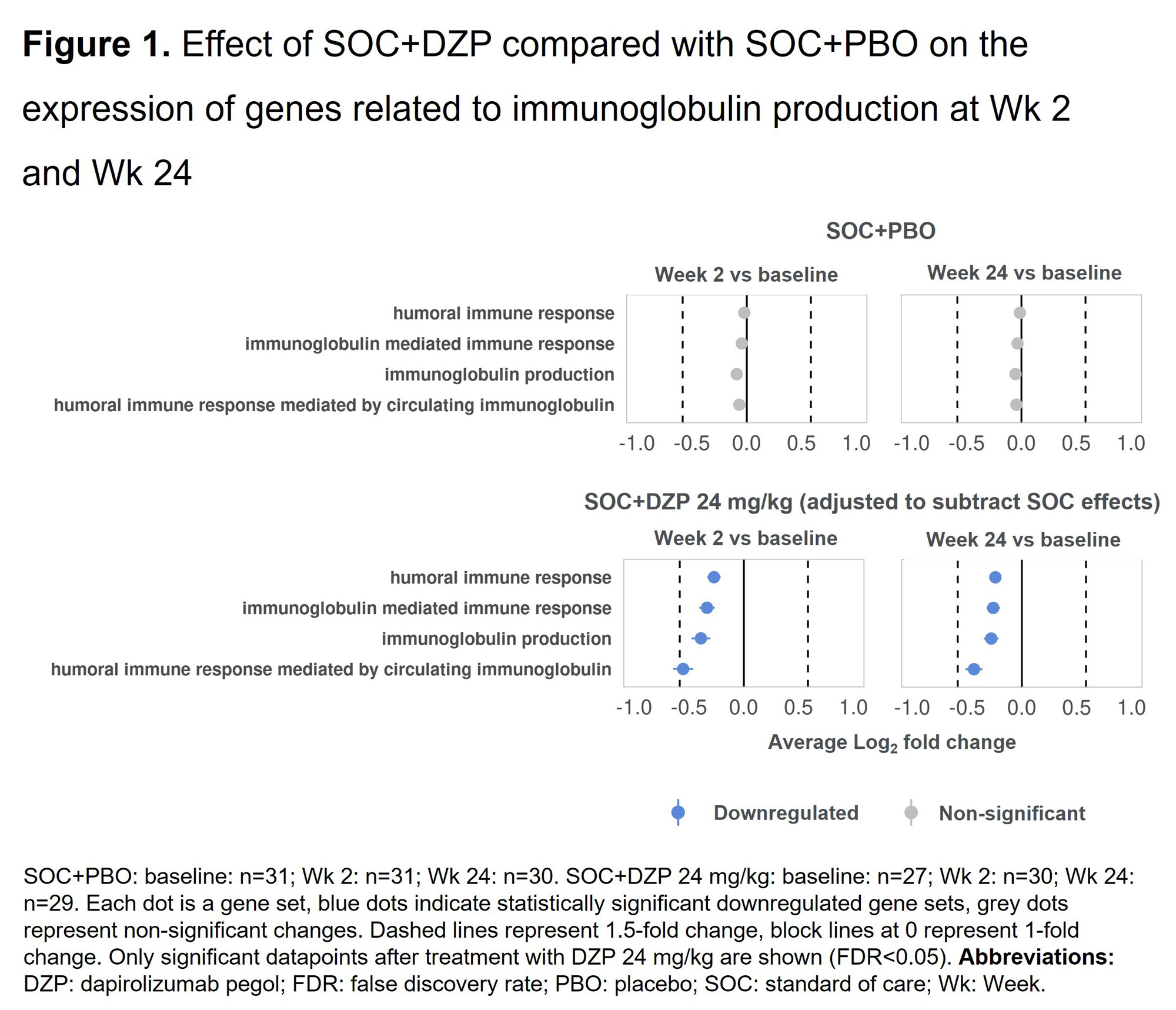
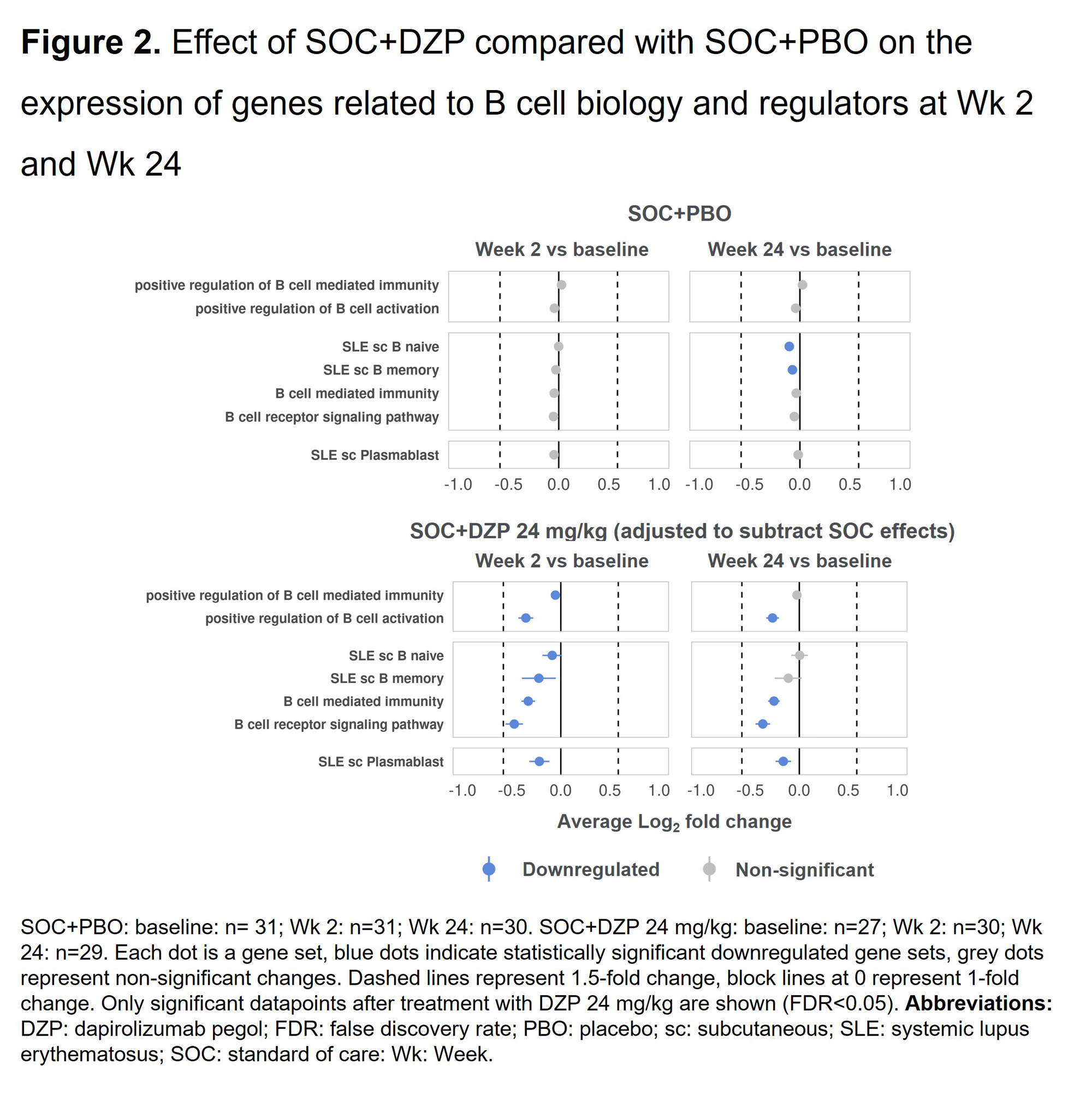
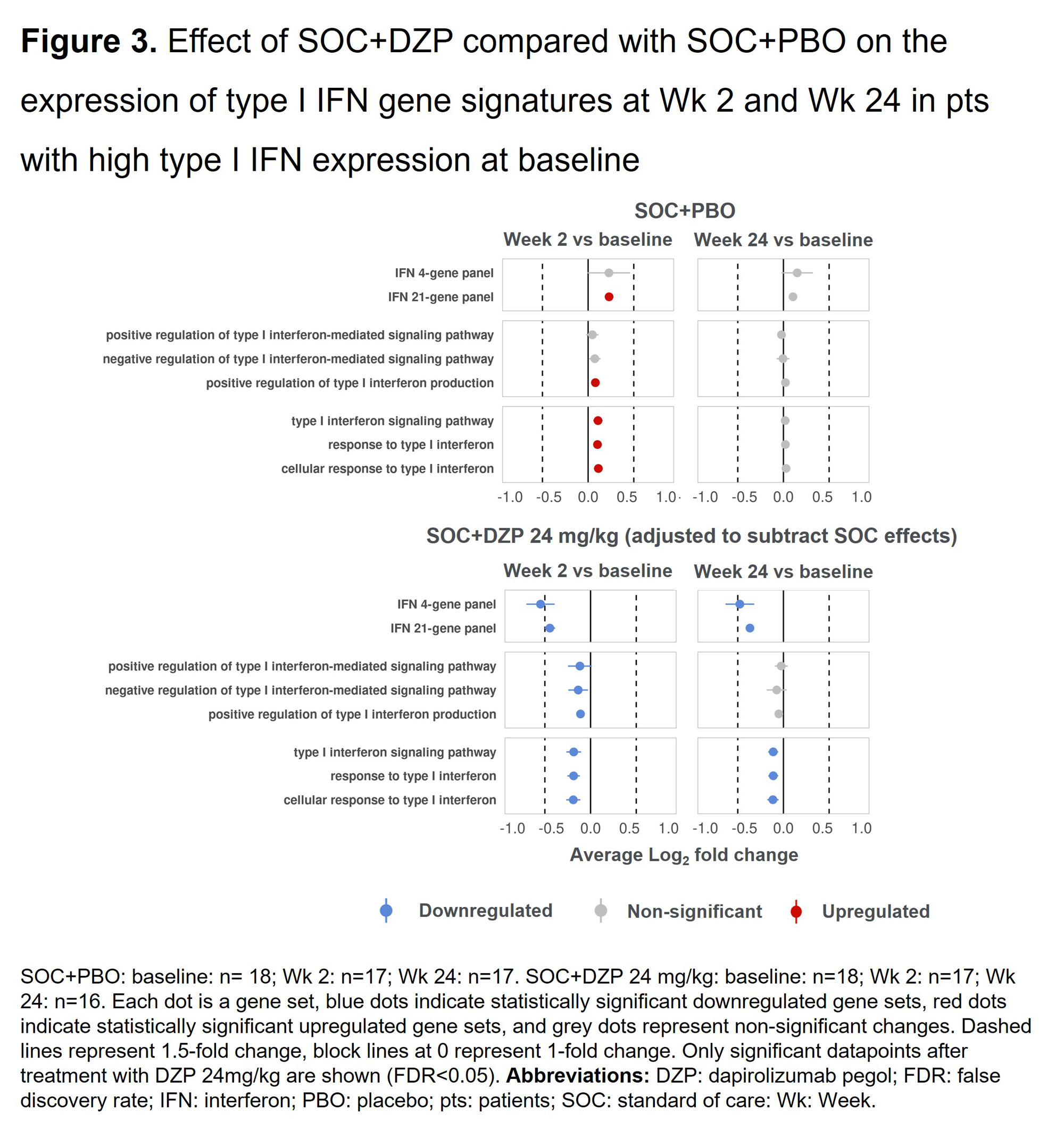
Results: DZP significantly downregulated genes related to immunoglobulin production compared with PBO, and this effect was observed as early as Wk 2 following a single dose (Figure 1). A similar trend was observed in clinical levels of autoantibodies (anti-dsDNA), which has been previously reported.3Compared with PBO, DZP also significantly downregulated genes that play a critical role in B cell biology, specifically those involved in B cell mediated immunity and B cell receptor signaling pathways (Figure 2). At baseline, 76/120 pts (63.3%) with RNAseq samples available across all treatment arms showed high type I IFN gene expression. In pts with high expression, DZP caused down-modulation of a variety of type I IFN gene signatures compared with PBO, with inhibitory effects observed as early as Wk 2 (Figure 3).
Conclusion: DZP exerts a broad suppressive effect on B cell biology, decreasing the expression of numerous gene sets involved in B cell activation and immunoglobulin production. Moreover, DZP decreases expression of type I IFN signatures in pts with high baseline type I IFN expression. The impacts of DZP were seen as early as Wk 2. These findings underscore the potent immunomodulatory role of DZP in SLE pathology.
References:1. Ramanujam M. Autoimmun Rev. 2020;19(11):102668. 2. Rönnblom L. Lupus Sci Med. 2019;6(1):e000270. 3. Furie RA. Rheumatology (Oxford). 2021;60:5397–407. 4. Askanase A. Ann Rheum Dis. 2023;82(Suppl 1):272. 5. Wu D. Nucleic Acids Res. 2012;40(17):e133. 6. Mandric I. Nat Commun. 2020;11(1):5504. 7. Felten R. Drug Des Devel Ther. 2019;13:1535–43.
To cite this abstract in AMA style:
Cutcutache I, Powlesland A, Skelton A, Sun Y, Page M, Stojan G, Lipsky P, Skowera A, Stach C. Dapirolizumab Pegol Impacts Important Immunologic Pathways in SLE: Pharmacodynamic Analysis of B Cell and Type I Interferon Pathways from a Phase 2b Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/dapirolizumab-pegol-impacts-important-immunologic-pathways-in-sle-pharmacodynamic-analysis-of-b-cell-and-type-i-interferon-pathways-from-a-phase-2b-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dapirolizumab-pegol-impacts-important-immunologic-pathways-in-sle-pharmacodynamic-analysis-of-b-cell-and-type-i-interferon-pathways-from-a-phase-2b-trial/