Session Information
Date: Tuesday, October 28, 2025
Title: (2437–2469) Systemic Lupus Erythematosus – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: SLE imposes significant disease burden and diminishes health-related quality of life (HRQoL); improvement of HRQoL is therefore a key treatment goal in SLE.1,2 Dapirolizumab pegol (DZP) is a novel CD40L inhibitor with broad modulatory effects on SLE immunopathology;3,4 it consists of a polyethylene glycol (PEG)-conjugated antigen-binding fragment (Fab’), which lacks an Fc domain. In the phase 3 PHOENYCS GO trial (NCT04294667), the primary endpoint was met; DZP plus standard of care (DZP+SOC) resulted in significantly higher rates of BILAG-based Composite Lupus Assessment (BICLA) response vs placebo (PBO)+SOC at Week (Wk) 48.5 We report the impact of DZP on HRQoL as measured by LupusQoL, completed by patients, in PHOENYCS GO.
Methods: PHOENYCS GO, a 48-wk, randomized, double-blind, PBO-controlled trial, included patients aged ≥16 years with moderate-to-severe, active SLE characterized by persistently active or frequently flaring/relapsing-remitting disease activity despite stable SOC medication (antimalarials, glucocorticoids and/or immunosuppressants). Patients were randomized 2:1 to intravenous DZP 24 mg/kg+SOC or PBO+SOC every 4 wks. HRQoL outcomes were measured using LupusQoL, a patient-reported outcome based on responses on a 5-point Likert scale to 34 items across 8 domains.1 Each domain score ranges from 0 to 100; higher scores indicate better HRQoL. The least square (LS) mean change from baseline in LupusQoL domain scores at Wk 12, 24, 36, and 48 are reported.
Results: In total, 97.6% (203/208) of patients receiving DZP+SOC and 95.3% (102/107) receiving PBO+SOC had LupusQoL responses available at any visit. Baseline LupusQoL scores were comparable between the treatment groups (Table). Patients receiving DZP+SOC demonstrated consistently greater improvements from baseline over time in LupusQoL scores across all domains compared with PBO+SOC (Figure). Patients receiving DZP+SOC reported greater improvements in the ‘Fatigue’ and ‘Burden to others’ domains at all assessed visits compared with those receiving PBO+SOC, as of Wk 12 (all nominal p < 0.05). Additionally, greater improvements were reported for patients receiving DZP+SOC compared with PBO+SOC in the ‘Physical health’ and ‘Planning’ domains at Wks 24, 36, and 48, in the ‘Pain’ and ‘Emotional health’ domains at Wks 36 and 48, in the ‘Intimate relationships’ domain at Wks 24 and 36, and in the ‘Body image’ domain at Wk 48 (each nominal p < 0.05).
Conclusion: Improvements in HRQoL were greater in patients treated with DZP+SOC compared with PBO+SOC across all LupusQoL domains, starting at the earliest time point (Wk 12) for some domains. These data, along with the previously reported significant improvements in overall disease activity,5 support the potential of DZP as a valuable treatment option in SLE to improve HRQoL.References: 1. McElhone K. Arthritis Care Res 2007;57:972–9; 2. Fanouriakis A. Ann Rheum Dis 2019;78:736–45; 3. Cutcutache I. Arthritis Rheumatol 2023;75 (suppl 9); 4. Powlesland A. Annals Rheum Dis 2024;83 (suppl 1):261; 5. Clowse M. Arthritis Rheumatol 2024;76 (suppl 9).
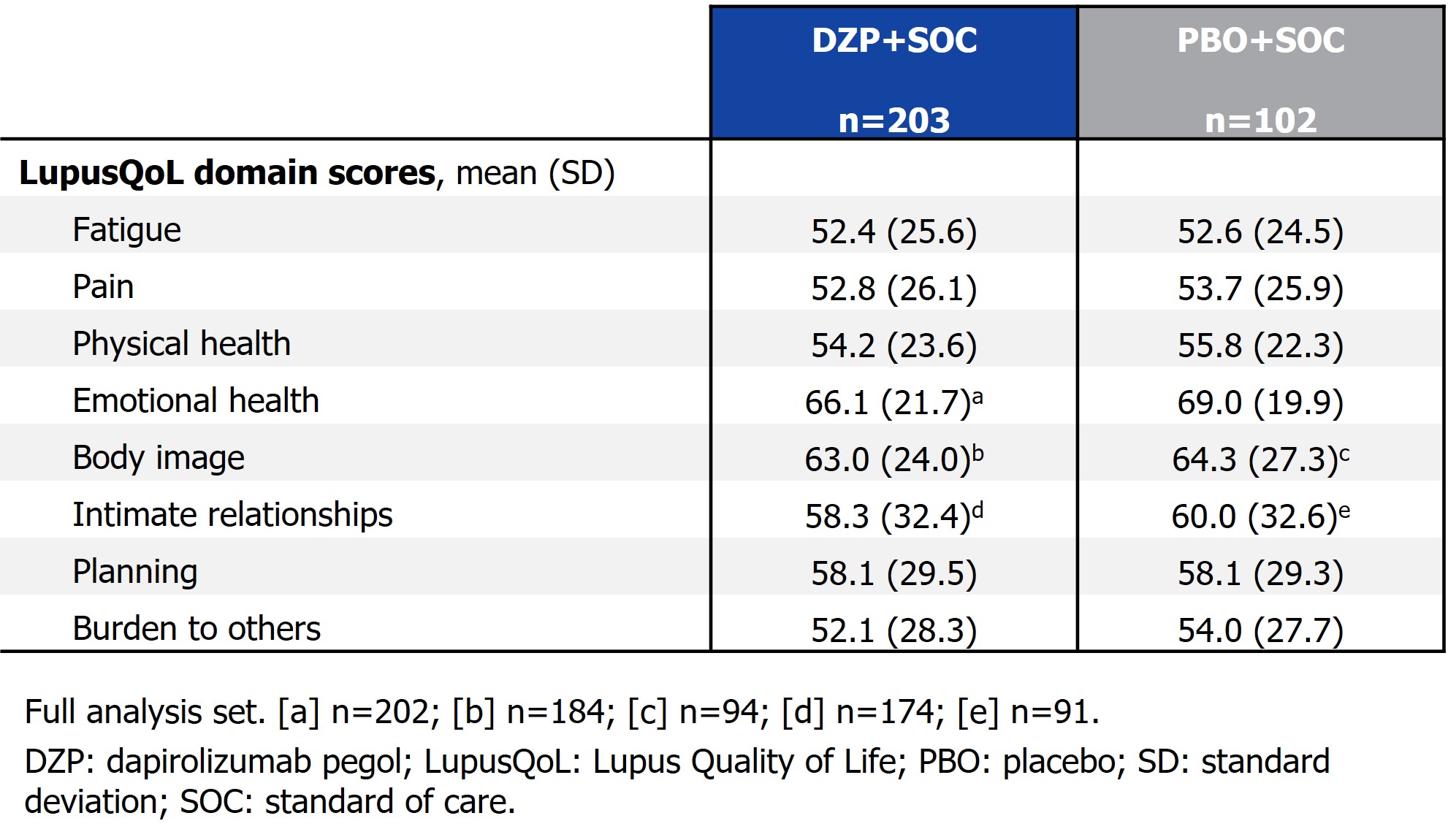 Table. Baseline LupusQoL domain scores
Table. Baseline LupusQoL domain scores
.jpg) Figure. LS mean change from baseline in LupusQoL domain scores by visit
Figure. LS mean change from baseline in LupusQoL domain scores by visit
To cite this abstract in AMA style:
Touma Z, Aranow C, Parodis I, Ramsey-Goldman R, Schneider M, de La Loge C, Jimenez T, Nejati M, arnaud L. Dapirolizumab Pegol Demonstrated Improvement in Quality of Life of Patients with Systemic Lupus Erythematosus: LupusQoL Results from a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/dapirolizumab-pegol-demonstrated-improvement-in-quality-of-life-of-patients-with-systemic-lupus-erythematosus-lupusqol-results-from-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dapirolizumab-pegol-demonstrated-improvement-in-quality-of-life-of-patients-with-systemic-lupus-erythematosus-lupusqol-results-from-a-phase-3-trial/
