Session Information
Date: Monday, November 18, 2024
Title: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis (RA) is recognized as a risk factor for osteoporosis. Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) acts as an inhibitory regulator post-T cell activation, attenuating the antigen-specific immune response. However, the influence of Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) on osteoporosis among RA patients remains unexplored. This study aims to investigate the efficacy of the CTLA-4 agonist, abatacept, in arresting bone loss in RA patients and to analyze its impact on bone turnover markers, thereby elucidating its potential role in mitigating osteoporosis associated with RA.
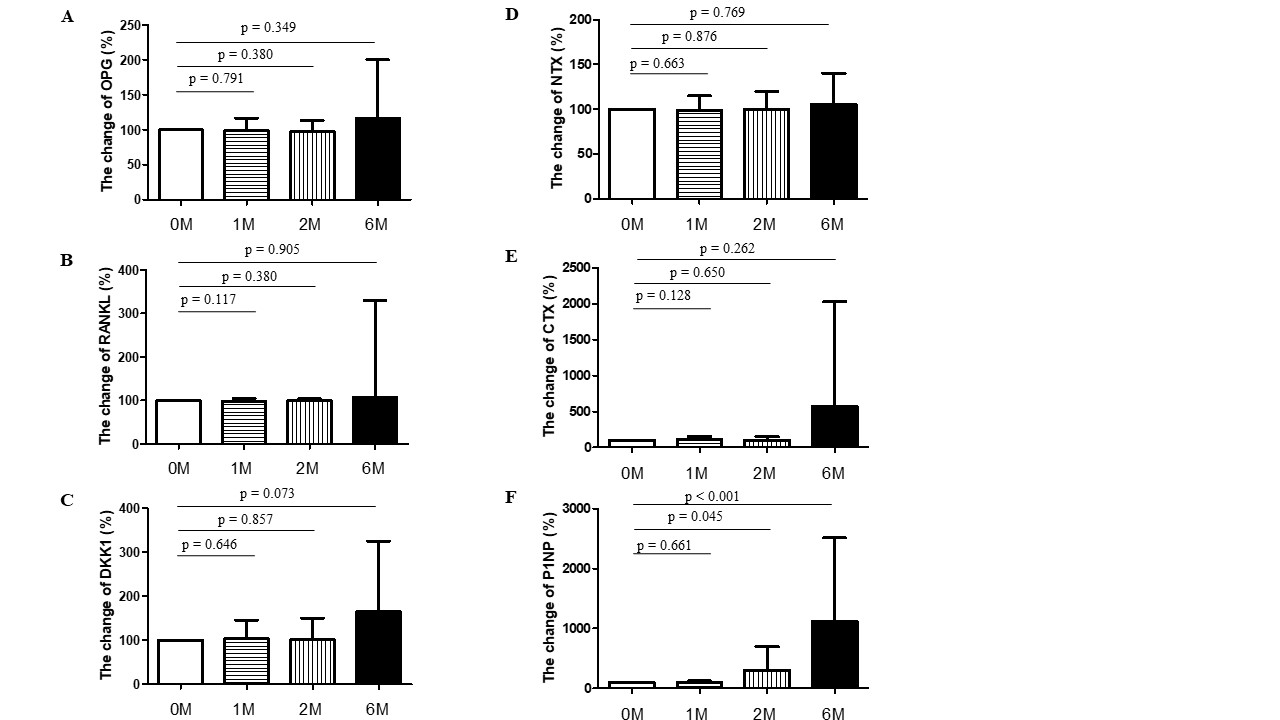
Methods: We prospectively analyzed bone mineral density (BMD) of RA patients undergoing CTLA-4 fusion protein (abatacept) treatment. Dual-energy x-ray absorptiometry (DXA) scan was used to measure areal BMD at lumbar spine and femur. Clinical and laboratory assessments were conducted for patients with rheumatoid arthritis (RA), including age, sex, body mass index, rheumatoid factor (RF), and anti-cyclic citrullinated peptide antibodies (ACPA). Additionally, bone turnover markers such as osteoprotegerin (OPG), receptor activator of nuclear factor kappa-B ligand (RANKL), Dickkopf-1 (DKK1), C-terminal telopeptides of type I collagen (CTX), N-terminal telopeptides of type I collagen (NTX), and procollagen type I N-terminal propeptide (P1NP) were quantified using enzyme-linked immunosorbent assays (ELISA).
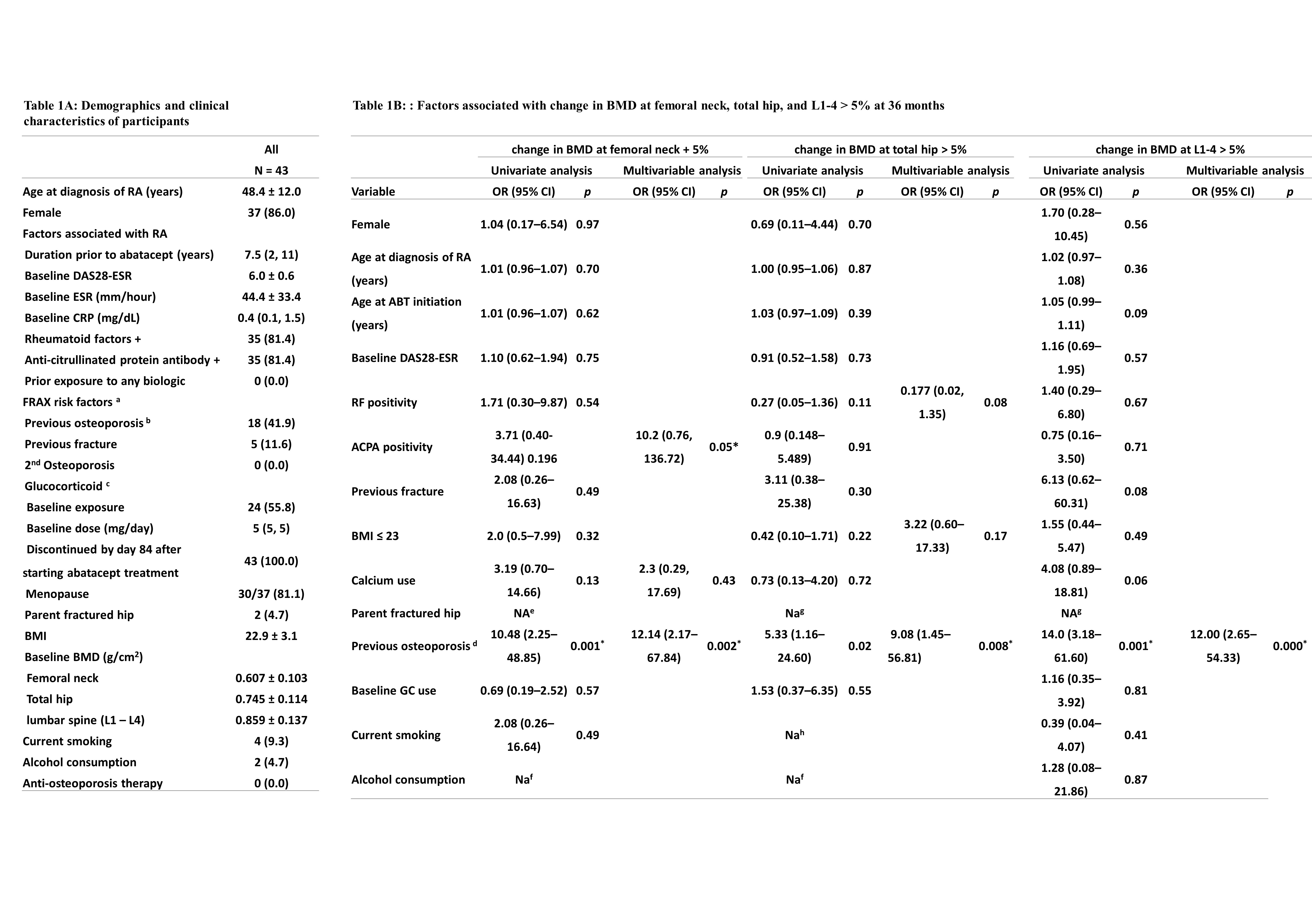
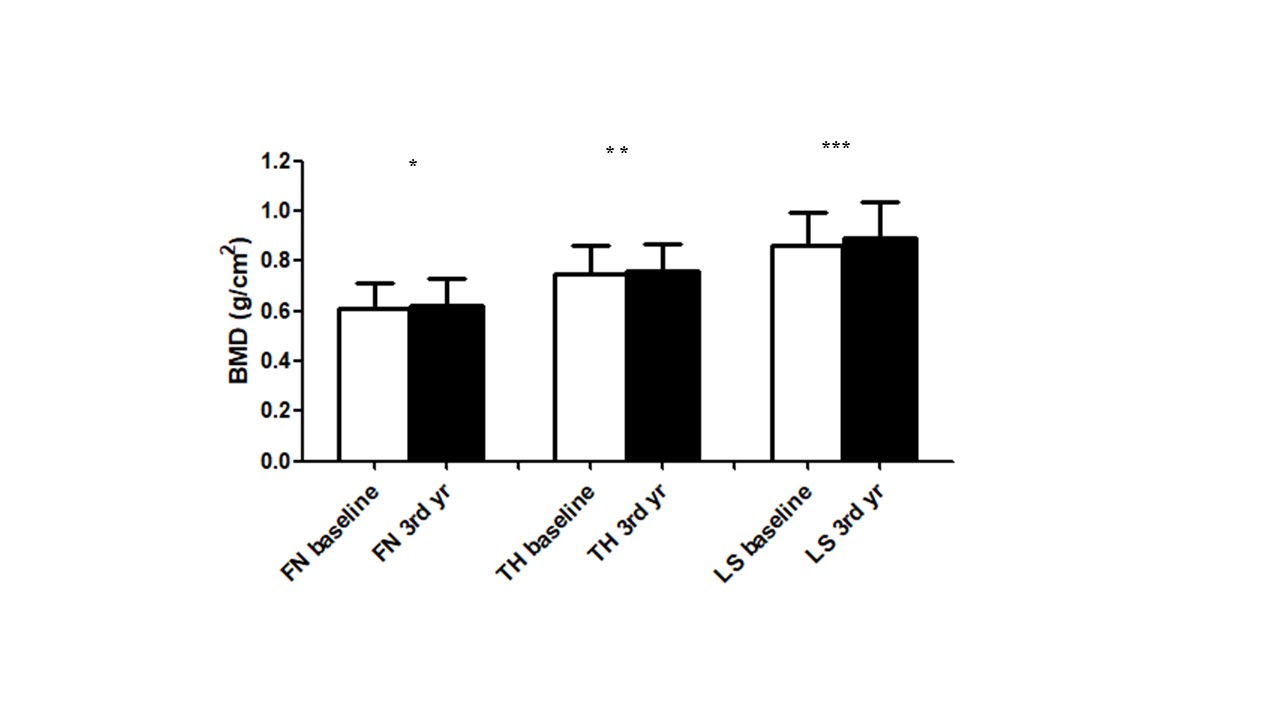
Results: A total of 43 RA patients participated in this study. Baseline demographics were listed in Table 1A. Notably, abatacept treatment was associated with significant increases in BMD at the femoral neck, total hip, and lumbar vertebrae 1–4 over a period of three years (p = 0.025, 0.010, and 0.001, respectively) (Figure 1). Interestingly, patients with a history of osteoporosis exhibited greater increases in BMD at the femoral neck (p=0.032) and lumbar vertebrae L1–L4 (p=0.006). Enhanced BMD at the total hip was observed in patients who commenced abatacept treatment after the age of 50 years (p=0.003), those who were menopausal (p=0.006), and those with a body mass index (BMI) over 23 (p=0.037) (Table 1B). A significant increase in serum P1NP levels, indicative of bone formation, was detected after six months of abatacept therapy (p < 0.001). Conversely, no significant changes were observed in serum levels of OPG, RANKL, DKK1, NTX, and CTX (all p > 0.005) (Figure 2).
Conclusion: Long-term abatacept treatment for 3 years may offer a protective effect against bone loss in patients with RA. Additionally, patients predisposed to osteoporosis may experience greater benefits from abatacept. It is hypothesized that the primary mechanism by which CTLA-4 protects against bone loss is through the enhancement of bone formation. Further research is needed to elucidate the mechanisms underlying CTLA-4-mediated upregulation of osteogenesis.
Table 1A: Demographics and clinical characteristics of participants
Data are presented as frequency (percentage), mean ± standard deviation, or median (interquartile range).
a, defined as in FRAX tool
b, defined as a T-score equal to −2.5 or less at femoral neck
c, dose of glucocorticoid was converted to a prednisolone equivalent dose
Table 1B: Factors associated with change in BMD at femoral neck, total hip, and L1-4 > 5% at 36 months
d, defined as a T-score equal to −2.5 or less at femoral neck
e, None achieved change in BMD at femoral neck > 5% in patients whose parents had fracture.
f, None achieved change in BMD at femoral neck and total hip > 5% in patients with alcohol consumption.
g, Both achieved change in BMD at total hip and L1 – 5 > 5% in two patients whose parents had fracture.
h, None achieved change in BMD at total hip > 5% in patients with smoking habit.
*P < 0.05
* p < 0.05 **p < 0.01 *** p < 0.001
Abbreviations: 3rd, third; yr, year.
To cite this abstract in AMA style:
Chang Y, Chen M. Cytotoxic T Lymphocyte Antigen-4 Improves Osteoporosis in Patient with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cytotoxic-t-lymphocyte-antigen-4-improves-osteoporosis-in-patient-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cytotoxic-t-lymphocyte-antigen-4-improves-osteoporosis-in-patient-with-rheumatoid-arthritis/